
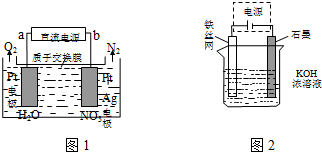
 ������������Ҫ�Ĺ�ҵ��Ʒ����ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ����ش�
������������Ҫ�Ĺ�ҵ��Ʒ����ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ����ش����� ��1���������ӽ���Ĥ��ⷨ��ȥ��ҵƷ����������Һ�е����ʺ���������൱�ڵ��ˮ���������������ӷŵ磻
��2�����ʱ������H+�ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ�������ƶ��ٽ�ˮ�ĵ��룬OH-Ũ������
��3��������������֮���������ӽ���Ĥ��ֻ����������K+��H+ͨ�������������������ۼ�������K+��OH-���Ӷ�������������������Һ�����Գ�ȥ���ʺ������������Һ����Һ��������������
��� �⣺��1���������ӽ���Ĥ��ⷨ��ȥ��ҵƷ����������Һ�е����ʺ���������൱�ڵ��ˮ����������������Ӧ���ʵ��ʱ�����缫��Ӧʽ��4OH--4e-=2H2O+O2����
�ʴ�Ϊ��4OH--4e-=2H2O+O2����
��2�������缫��Ӧʽ��4H++4e-=2H2��������������H+�ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ�������ƶ��ٽ�ˮ�ĵ��룬��Һ��OH-Ũ����������������ҺpH������
�ʴ�Ϊ��H+�ŵ磬�ٽ�ˮ�ĵ��룬��Һ�е�OH-Ũ������
��3�����������ۼ�������K+��OH-���Ӷ�������������������Һ�����Ӻ������������Һ�ӳ���B������
�ʴ�Ϊ����ȥ���ʺ������������Һ��
���� ���⿼����ԭ����Ӧ�ã��ؼ��Ǹ������ӷŵ�˳���жϵ������з����ķ�Ӧ���������ԭ������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4 | B�� | NaOH | C�� | Na2SO4 | D�� | NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
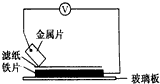 ���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ�������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������±���ʾ˵����ȷ����D
���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ�������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������±���ʾ˵����ȷ����D| A�������ֽ�����C�Ļ�ԭ������ |
| B������B�ܴ�����ͭ��Һ���û���ͭ |
| C��AD���γ�ԭ���ʱAΪ���� |
| D��AB�γɺϽ�ʱ�����úϽ�¶���ڿ����У�A�ȱ���ʴ |
| ���� | ������������ | ��ѹ/V |
| A | A��Cu | +0.78 |
| B | Cu��B | +0.15 |
| C | C��Cu | +1.35 |
| D | D��Cu | +0.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��ͼ��ʾ��
�ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�����մɵ���Ҫ�ɷ־��ǹ����� | |
| B�� | SO2����ʹ��ˮ��ɫ������SO2����Ư���� | |
| C�� | ��Ũ��ˮ�ε��������ƹ����п�����ȡ���� | |
| D�� | NaHCO3����Ӧ�����ʳƷ��ҵ���������Ƹ������ɼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O | B�� | ���� | C�� | NaOH��s�� | D�� | Na2SO4��s�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Ӧ���ʽ��� | B�� | ƽ�������ƶ� | C�� | B��ת������� | D�� | a+b��c+d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ������
��Cl2����ijЩ�����л���ʱ�����������HC1������ ��ӦA����ʵ���ȵ�ѭ�����á�
��ӦA����ʵ���ȵ�ѭ�����á�
��ӦA��4HCl��O22Cl2��2H2O
��1����֪:��ӦA�У� 4mol HCl���������ų�115.6kJ��������
4mol HCl���������ų�115.6kJ��������
��
�ٷ�ӦA���Ȼ�ѧ����ʽ��________________________��
�ڶϿ�1 mol H��O ����Ͽ� 1 mol H��Cl �������������ԼΪ__________kJ��H2O��H��0 ����HCl��H��Cl�����ǿ����������_______________��
��2���ϳɰ�������ͨ���ⶨ��Ӧǰ����������ܶ���ȷ������ת���ʡ�ij������úϳ�����N2��H2���������ܶ�Ϊ0.5536g/L����״�������Ӻϳ����г����Ļ����������ͬ�������ܶ�Ϊ0.693g/L����״�������úϳɰ���N2��ת����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��һ�����������ۡ�8•12���ش���ֱ�ը�¹ʣ���һ�����������ǶԻ�������Ĺ�ע��
��һ�����������ۡ�8•12���ش���ֱ�ը�¹ʣ���һ�����������ǶԻ�������Ĺ�ע���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com