
��13�֣���֪HI��һ����ɫ���д̼�����ζ����������ˮ�����壬HI��ˮ��Һ��֮Ϊ����ᣬ��һ��ǿ�ᡣ
��1����д����Ԫ�������ڱ���λ�ã��������� �塣
��2����HI����ͨ��һ������Ũ�����У������Ļ���������HI��������I2������ˮ�����⣬������
�� ���塣
��3��С�����HIͨ��Ũ�����Ļ������ɷֽ�����֤��̽���������������ʵ��װ��ͼ����̽����ƣ�
������ֱ�β���������װ��ҩƷ�� ��д���ƣ�
���������Ȼ�̼�����������ǣ� �� ��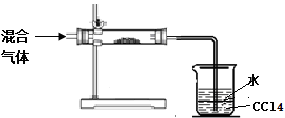
��һ��̽����
�������ϣ�������ǿ��KMnO4>HNO3>I2>SO42�����ҽ�ϡ�����������I2��
��С���������ˮ��������������ɷ�����һ��̽���������������ѡ�Լ���ѡ����ʵ��Լ���С�����ʵ�鱨�档
��ѡ���Լ���ʯ���Լ���Ʒ����Һ������KMnO4��Һ��0.1mol/L HNO3��������Һ��BaCl2��Һ
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ȡ�����ձ��е��ϲ���Һ��װ��A��B��֧�Թ��� | |
| | |
| | |
��1�� VIIA��1�֣� ��2�� SO2��2�֣�
��3�� ����ˮ����ͭ��2�֣� �ڼ��顢���յ���������������3�֣�ÿ��1�֣� �ۣ��𰸺�������֣�ʵ�鷽�� ���ܵ��������Ӧ�Ľ��� ��A�Թ��м�������Ʒ����Һ��1�֣� ��Ʒ����Һ��ɫ����ԭ�����������SO2
����Һ����ɫ����ԭ���������û��SO2��1�֣���B�Թ��еμ�����������Һ��1�֣����ٵ�������������Һ��1�֣� ��������Һ��������ԭ�����������HI
����Һ����������ԭ���������û��HI��1�֣�
���������������1�����ԭ��������53����˵�Ԫ�������ڱ���λ���ǵ������ڵ�VIIA�塣
��2������Ũ������������ԣ����⻯����л�ԭ�ԣ��ܱ�Ũ�����������ɵ��ʵ⣬���ᱻ��ԭΪSO2�����Խ�HI����ͨ��һ������Ũ�����У������Ļ���������HI��������I2������ˮ�����⣬��������SO2���塣
��3������������ͨ����Һʱ�����ˮ�������������ȼ���ˮ������һ������ˮ����ͭ��������ֱ�β���������װ��ҩƷ����ˮ����ͭ��
�ڵ��ܱ����Ȼ�̼��ȡ������Ϊ�⻯�⼫������ˮ�������������Ȼ�̼�����������Ǽ��顢���յ�����������������á�
�������ϲ���Һ���ܽ���ǵ⻯���SO2������SO2������Ʒ����Һ������Ϊ������ǿ��KMnO4��HNO3��I2��SO42�����ҽ�ϡ�����������I2�����Լ��������Ӧ����ϡ���ᣬ���ʵ�鷽�������Ϊ
���㣺������������ʵ�鷽�������̽��ʵ�鷽�� ���ܵ��������Ӧ�Ľ��� ��A�Թ��м�������Ʒ����Һ��1�֣� ��Ʒ����Һ��ɫ����ԭ�����������SO2
����Һ����ɫ����ԭ���������û��SO2��1�֣���B�Թ��еμ�����������Һ��1�֣����ٵ�������������Һ��1�֣� ��������Һ��������ԭ�����������HI
����Һ����������ԭ���������û��HI��1�֣�


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ϊ98%���ܶ�Ϊ1��84 g��cm��3��ŨH2SO4������500 mL 0��2 mol��L��1��ϡH2SO4���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� ��ҩ�� ����Ͳ ������ƿ ��������ƽ ����ش��������⣺
��1�����������У�������ϡH2SO4ʱ��Ƿȱ�������� ��
��2�������㣬��ŨH2SO4�����Ϊ mL����ȷ��0��1����
��3�����ƹ��������²�����
A����Һ
B����ȡ
C��ϴ��
D������
E��ϡ��
F��ҡ��
����ȷ�IJ���˳��Ӧ�� (�����)��
��4�������ƹ����У�����������ȷ�����в����У����������ƫ�ߵ��� (�����)��
A������ʱ�����ӱ���
B��ϡ�ͺ��H2SO4��Һδ����ȴ�����¾�ת�Ƶ�����ƿ��
C��ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ������
D��ת��ǰ������ƿ�к�����������ˮ
��5������������ƿ����ȡ25��00mL��ϡ������Һ��100mL������ƿ����ˮϡ�����̶��ߡ�����������Һ��c��H+��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�õ������壨CuSO4��5H2O ������0.040mol/L��CuSO4��Һ240mL���ش��������⣺
��1����������Ϊ��������ƽ��ҩ�ס��ձ�����Ͳ����ͷ�ιܣ�����Ҫ��Щ��������������ɸ�ʵ�飬��д���� ��
��2����д����ʵ��ļ�Ҫ��ʵ�鲽�裺
�ټ���ڳ������� g�� ��ת�Ƣ�ϴ�Ӳ�ת�Ƣ��ݢ�ҡ��
��3����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ����� ��
��4����ͬѧ�ڶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȡ���������ҺŨ�ȵ�Ӱ�� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5��һƿ���ƺõ�ϡCuSO4��Һ����ʱ���������ǩ����Ϊ����������ʢ��CuSO4��Һ����д������SO42-ʵ����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��20�֣���18.4mol��L-1��Ũ���ᣬ���� 100 ml 1.0 mol��L-1��ϡ���ᣬ��ش��������⣺
��1����Ҫ18.4mol��L-1��Ũ���� ml��
��2��������������������ʱ����Ҫ�õ� �� ��
��������ƽ �ڷ�Һ©�� ��250ml����ƿ ���ձ� �ݽ�ͷ�ι�
����Ͳ �߲�����������̨�������У� ��100ml����ƿ
A���ۢܢݢߢ� B���٢ڢݢޢ� C���٢ڢۢ� D���ۢܢݢ�
��3������ʵ�鲽���У���ȷ�IJ���˳��Ӧ���ǣ�
A ����Ͳ��ȡŨ���ᣬ��������װ��Լ50ml����ˮ���ձ�����ò��������衣
B ��Լ30ml����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴ��Һ����������ƿ�У�
C ��ϡ�ͺ������С�ĵص�������ƿ�У�
D.���100ml����ƿƿ���Ƿ���©Һ����
E.������ˮֱ�Ӽ�������ƿ����Һ��ӽ��̶���1����2cm����
F.�ǽ�ƿ���������ߵ���ҡ����Һ��
G.�ý�ͷ�ι�������ƿ����μ�������ˮ����Һ����͵���̶������У�
��4������A���������ʱ��Ӧ��ѡ��
��10 ml��Ͳ ��50 ml��Ͳ ��5000 ml��Ͳ ��1000 ml��Ͳ
��5������A���������� ���ܽ���C��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ÿС��2�֣����о������ʣ�1�����ߣ�2��ʯī��3��������4������þ���壨5�������ᣨ6�����ʯ��7��ʯ��ˮ��8���Ҵ���9�����ڵ������
�����ܵ������ �����ڵ���ʵ���
�Ȳ��ǵ����Ҳ���Ƿǵ���ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ϱ�������Ŀǰ�������н�20���˻���ȱ����ƶѪ��Ӱ�����˵����彡�������������и��Ͷ�ͯΣ���������ء����ҹ������������ˡ����Ͳ������̡������������ָ
| A����Ԫ�� | B�������� | C�������� | D������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��������������أ�����˵���������
| A���������ƿ�������Ա��DZˮԱ�Ĺ����� |
| B���ɱ��͵⻯�����������˹����� |
| C�����������ֵij�������������β�����ŷ��� |
| D����������ֲ��ϲ������Ͻ���Ϊ���Ͻ�ǿ�ȴ������ᡢ����ʴ����ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ����ͨ��һ����Ӧ�õ�����
| A��Na2CO3 �� NaHCO3 | B��SiO2 ��H2SiO3 |
| C��Fe(OH)2 �� Fe(OH)3 | D��NH4Cl �� NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��֪AΪ����ɫ���壬T��RΪ���ֳ�������;�ܹ�Ľ������ʣ�D�Ǿ��д��Եĺ�ɫ���壬C����ɫ��ζ�����壬H�ǰ�ɫ������W��Һ�м���KSCN����Ѫ��ɫ��
��1��д���������ʵĻ�ѧʽ��D�� E�� N�� ��
��2��B��E��͵õ�H���ڳ�ʪ�����б��M�Ĺ����У����ܹ۲쵽������
��3����Ҫ��д����ʽ��
B��R��Ӧ����N�����ӷ�ʽ�� ��
M��W�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com