
���и���ʵ��������������ó��Ľ����У���ȷ����
ѡ�� ʵ����������� ʵ�����
A �����ݵ�������Һ�зֱ�μ�NaCl��Һ��
CuSO4��Һ�����й������� �����ʾ������˱���
B ȡ����Fe��NO3��2������ˮ�ܽ⣬��ϡ�����ữ��
�Ρ���KSCN��Һ����Һ��Ϊ��ɫ ��Fe��NO3��2�����Ѿ�
����
C ������ij���ʵ���Һ�μӵ����Ƶ�������Һ�У�
ˮԡ���Ⱥ����������� ������һ������ȩ��
D ͬ�����£��ֱ�0.1mol��L��1������ʹ����
�е�����ʵ�飬����ᴮ���ĵ��ݽϰ� ����������
D
��������
��������� ������Һ�μ�NaCl��Һ���й���������ԭ���Ƿ�������������A�����Fe��NO3��2��NO3‾������ϡ�������Һ�ֺ��д���H+������Һ����HNO3, HNO3���Fe��NO3��2����Ϊ Fe��NO3��2������˵��ԭ�������ʣ���B�����������Ҳ�ܷ���������Ӧ���������Dz���ȩ����C�������ͬŨ�ȵ�����ʹ�����ͬ�����½��е�����ʵ�飬����ᴮ���ĵ��ݽϰ���˵���������̶�С��Ϊ���ᣬ��D����ȷ��
���㣺 ���⿼�鵰���ʡ�����������ԡ�������Ӧ���������Һ�ĵ����ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��.װ������װ���Լ���?
��Aƿװ��ˮ�Ҵ����ڷ���ˮ��X?
��B�������װ��ʯ��?
��C��D�ж�װŨ����?
��Eƿ��װ���Լ�Y?
��.ʵ�����������?
��ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д�������ð����Aƿ��X��ɫ����B�ܿڻӷ��������ȼ�ա�?
������������⣺?
��1��Eƿ����װ���Լ�Y�ǣ�������?
a.����ʳ��ˮ? b.MnO2��NaCl�Ļ����? c.Ũ����?
��2��D��Ũ���������������____________________��Cƿ��Ũ���������������____________________��
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��______________________________����Ӧ������__________�������ɵ�__________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��__________��������ָʾ���õ�ԭ����_________________________ _______________��?
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������____________________________��
��6�������װ���е�Cƿȡ����ʵ��Ŀ���Ƿ��ܴﵽ��__________����Ϊ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ڽ��и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij�о�С��̽����
I ��ͭƬ��Ũ����ķ�Ӧ���г�װ�ú�A�м���װ������,�������Ѽ��飩
II�� SO2 �� Fe( NO3)3 ��Һ�ķ�Ӧ[1.0 mol/L �� Fe(NO3)3 ��Һ�� pH��1] ��ش������й����⣺
̽��I
(l)ijѧ����������ʵ��:ȡ12.8gͭƬ��20 mL 18 mol•L-1��Ũ�����������ƿ�й���,ֱ����Ӧ ���,�������ƿ�л���ͭƬʣ�࣬ͬʱ������ѧ��֪ʶͬѧ����Ϊ���н϶������ʣ�ࡣ

��װ��A�з�Ӧ�Ļ�ѧ����ʽ��_______
�ڸ�ͬѧ���������Ũ�ȵ�ʵ�鷽���Dzⶨ��������������䷽���ж���,�������з����в����е���______ (����ĸ����
A�������������建��ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ����,������Ӧ���ٴγ��F
B�������������建��ͨ�������{�������Һ,�ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ������������
C������ˮ���ⶨ�������������(����ɱ�״����
D�����ű���NaHSO3��Һ�ķ����ⶨ�������������(����ɱ�״����
̽��II
(2)Ϊ�ų�������ʵ��ĸ��ţ��μ�Ũ����֮ǰӦ���еIJ�����______��
(3)�b��B�в����˰�ɫ����������B�в�����ɫ������ԭ������������ֲ��룺
����1:SO2��Fe3+��Ӧ������2 ��������������SO2��NO3-��Ӧ������3��____________��
�ٰ�����1��װ��B�з�Ӧ�����ӷ���ʽ��______��֤���ò���Ӧ��һ��ȷ�����ɵ������ʣ���ʵ�������������____________��
�ڰ�����2,ֻ�轫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�����������ij����Һ������ͬ�����½���ʵ�顣Ӧѡ����滻��Һ��______ (����ţ���
a��0.1 mol/L ϡ���� b�� 1.5 mol/L Fe(NO3)2 ��Һ
c�� 6.0 mol/L NaNO3��0.2 mol/L����������ϵ���Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��������찲�ص�����ѧ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣���һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֣������ӣ�S2- ��MnO4- ��NO3-��SO42-��CO32-�������ӣ�Na+��Al3+��Ba2+��Fe3+��NH4+������ɫ��ĩ��������ʵ�飬ʵ��������۲쵽�������£�
a��ȡ������ĩ��ˮ�������ǣ�ȫ���ܽ⣬��Һ��ɫ����
b����������Һ���������������Һ�����ȣ������ǣ�����������
c��ȡ������ĩ�����ᣬ�����ǣ�����������
d��ȡ������ĩ��ϡ�����ϡ������Һ�������ǣ��в�����ϡ����İ�ɫ�������ɡ�
����ʵ���ƶϣ�
��1����aʵ���п��ƶϷ�ĩ��һ��û��________���ӣ�
��2����bʵ���п��ƶϷ�ĩ��һ��û��________���ӣ�
��3����cʵ���п��ƶϷ�ĩ��һ��û��________���ӣ�
��4����dʵ���п��ƶϷ�ĩ�бض�����________���ӣ�
��5��������������ĩ�л����ܺ���________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��C2H5OH+HBr��C2H5Br+H2O��Ϊ֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�顣�Ը�������װ��ͼ���Լ���ʵ�����ش��й����⡣
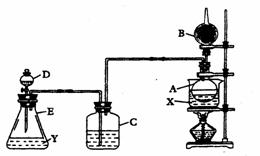 ��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��C��D�ж�װŨ�����Eƿ��װ���Լ�Y
ʵ������������ǣ�
��.��ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�ܿڻӷ�������ɵ�ȼ����ش����и����⣺
��1��Eƿ����װ���Լ�Y�����µ�???????________
A.����ʳ��ˮ�������� �� B.MnO2��NaCl�Ļ����������� C.Ũ����
��2��Dƿ��Ũ���������������_____�������� __ ;Cƿ��Ũ���������������________ ___�������������� ___
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��________________________________����Ӧ������____________�������ɵ�__________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��_________������ָʾ���õ�ԭ����__���������������� ______
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������__________________________________
(6)�����װ���е�Cƿȡ����ʵ��Ŀ���Ƿ��ܴﵽ________����Ϊ_____������ ___������������������������������ ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com