

 ��R��R������������
��R��R������������ ��
�� ��
�� ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOH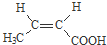 ��
�� ��
�� ���� �������и�����ת����ϵ����PMMA�Ľṹ��֪��PMMA����ΪCH2=C��CH3��COOCH3��E������F��F�ĺ˴Ź���������ʾֻ��һ��壬F������Ϣ���еķ�Ӧ��G��G��Ũ���������·�����ȥ��Ӧ��J�����PMMA����Ľṹ��E�ķ���ʽ��֪��EΪCH3CHOHCH3��FΪCH3COCH3��GΪ��CH3��2COHCOOH��JΪCH2=C��CH3��COOH������DΪHOCH3����ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br��A�ڼ���������ˮ���BΪHOCH2CH2OH��B��Ա��������������ȡ����Ӧ����PET����Ϊ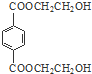 ��PET���巢����Ϣ��ķ�Ӧ��PET�������ݴ˴��⣮
��PET���巢����Ϣ��ķ�Ӧ��PET�������ݴ˴��⣮
��� �⣺�������и�����ת����ϵ����PMMA�Ľṹ��֪��PMMA����ΪCH2=C��CH3��COOCH3��E������F��F�ĺ˴Ź���������ʾֻ��һ��壬F������Ϣ���еķ�Ӧ��G��G��Ũ���������·�����ȥ��Ӧ��J�����PMMA����Ľṹ��E�ķ���ʽ��֪��EΪCH3CHOHCH3��FΪCH3COCH3��GΪ��CH3��2COHCOOH��JΪCH2=C��CH3��COOH������DΪHOCH3����ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br��A�ڼ���������ˮ���BΪHOCH2CH2OH��B��Ա��������������ȡ����Ӧ����PET����Ϊ ��PET���巢����Ϣ��ķ�Ӧ��PET������
��PET���巢����Ϣ��ķ�Ӧ��PET������
��1����������ķ�����֪����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ��
��2���ڵĻ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��3��PMMA����ΪCH2=C��CH3��COOCH3��PMMA����Ĺ�����������̼̼˫���� ������
�ʴ�Ϊ��̼̼˫����������
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��a����ΪCH2=C��CH3��COOH��HOCH3����������Ӧ����a��ȷ��
b��DΪHOCH3��BΪHOCH2CH2OH�����ǵ��ǻ�����Ŀ��ͬ������B��D���ǻ�Ϊͬϵ���b����
c��D�����ǻ������γ����������D�ķе��̼ͬԭ�����������ߣ���c��ȷ��
d.1mol  ������NaOH��Һ��Ӧʱ���������2mol NaOH����d����
������NaOH��Һ��Ӧʱ���������2mol NaOH����d����
��ѡac��
��6��J��ij��ͬ���칹����J������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ��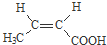 ���ʴ�Ϊ��
���ʴ�Ϊ��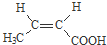 ��
��
��7����PET�����Ʊ�PET����������B�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ���ϳɣ�ע����ݳ������������Ϣ���л���Ľṹ�����ƶϣ���ȷ�л���Ĺ����ż��������ǽⱾ��ؼ����Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �٢ܢ� | C�� | �ڢۢ� | D�� | �ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ƽʱӦ�����úܴ�����ˮ����ֹ�ж����ʽ������� | |
| B�� | ������Ȼˮ���������� | |
| C�� | �����ں���Լ$\frac{2}{3}$���ص�ˮ������ÿ�첻�ú�ˮҲ�� | |
| D�� | ��������õ�ˮֻռ��Ȼ���ˮ������������Ӧ��Լ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������Ӻ������Ӹ�����һ����� | |
| B�� | ������һ�������Ӽ������й��ۼ� | |
| C�� | ��Xֻ������Ԫ�أ�����Ԫ��һ������ͬһ����Ҳ����ͬһ���� | |
| D�� | �����������Ӱ뾶һ�����������Ӱ뾶 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ��̼���ڼ�����ʽ������ũ���������������֪ʶ | |
| B�� | �û���̿Ϊ�ǽ���ɫ���ô�������Ư��ֽ����ԭ����ͬ | |
| C�� | �ȴ������ȥ���ۣ��������Ծ���ˮ��Ư�ۿ�����Ư��֯�� | |
| D�� | ��ˮ�����ķ������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S��g��+O2��g���TSO2��g����H��-297.3kJ•mol-1 | |
| B�� | 2SO2��g���T2S��s��+2O2��g����H=+297.3kJ•mol-1 | |
| C�� | 1molSO2�ļ����ܺ�С��1molS��1molO2�ļ����ܺ� | |
| D�� | 1molSO2�������������1molS��1molO2�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com