
 ��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�250mL 0.1mol?L-1��������Һ��| 1000��w |
| M |
| n |
| V |
| 1000��w |
| M |
| 1000��1.19��37% |
| 36.5 |
| 0.1mol/L��0.25L |
| 12.1mol/L |
| n |
| V |
| n |
| V |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������
������ �����ٶ����Ծ�����ͨϩ�������ʣ���һ���Ӹá�������ϩ���ڴ�����������һ����H2�����ӳɷ�Ӧ�������ӵĽṹ��ʽ��
�����ٶ����Ծ�����ͨϩ�������ʣ���һ���Ӹá�������ϩ���ڴ�����������һ����H2�����ӳɷ�Ӧ�������ӵĽṹ��ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��14�֣���2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g���T2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��14�֣���2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g���T2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��N O����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
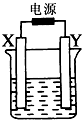 ��ͼװ����X��Y��Ϊʯī�缫�����ҺΪ500mLij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ�Y�缫����ɫ�������ɣ���Һ��ԭ��������ȫ����ֹͣ��⣬ȡ��X�缫��ϴ�ӡ�����������缫����1.6g�������й�˵���в���ȷ���ǣ�������
��ͼװ����X��Y��Ϊʯī�缫�����ҺΪ500mLij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ�Y�缫����ɫ�������ɣ���Һ��ԭ��������ȫ����ֹͣ��⣬ȡ��X�缫��ϴ�ӡ�����������缫����1.6g�������й�˵���в���ȷ���ǣ�������| A��X�缫������ |
| B��Y�缫������������Ϊ0.224L |
| C��������Һ��pH=1 |
| D��Ҫʹ������Һ�ָ������ǰ��״̬�������һ������CuO��CuCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaHCO3+NaHS��Na2CO3+H2S�� |
| B��H2S+Na2CO3��NaHS+NaHCO3 |
| C��Na2S+H2O+CO2��Na2CO3+H2S�� |
| D��H2S+NaHCO3��NaHS+H2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 X��Y��Z��WΪ������Ԫ�أ����������ڱ���λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵���д�����ǣ�������
X��Y��Z��WΪ������Ԫ�أ����������ڱ���λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵���д�����ǣ�������| A��X���⻯���ˮ��Һ�Լ��� |
| B��Y��Zֻ�����һ�ֻ����� |
| C��Z���������Z���⻯��ֱ�����ˮ����Һ�������� |
| D������������Ӧˮ��������ԣ�W��Z |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com