
һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1)��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
��NO3-�Ŀռ乹���� (����������)��
(2)��ͭ��������Ĵ��£�CO ������ΪCO2��HCHO ������ΪCO2��H2O��
�ٸ��ݵȵ�����ԭ����CO ���ӵĽṹʽΪ ��
��H2O ������O ԭ�ӹ�����ӻ�����Ϊ ��
��1 mol CO2 �к��еĦҼ���ĿΪ ��
��10�֣���1����1s22s22p63s23p63d5(��[Ar]3d5) ��2�֣� ��ƽ�������� ��2�֣�
��2����C��O ��2�֣� ��sp3 ��2�֣� ��2��6. 02��1023��(��2NA) ��2�֣�
��������
�����������1���ٸ��ݹ���ԭ����֪��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ1s22s22p63s23p63d5(��[Ar]3d5)��
��NO3-������ԭ�Ӻ��еŶԵ��Ӷ�������5��1��3��2����2��0��������ռ乹����ƽ�������Ρ�
��2����ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬���CO��Ϊ�ȵ�������ǵ��������������к�����������CO�Ľṹ��ʽ����C��O��
��ˮ��������ԭ�Ӻ���2�Թ¶Ե��ӣ���V�νṹ�������ԭ�ӵ��ӻ�������sp3�ӻ���
��CO2�����к���2��̼��˫������˫������1���Ҽ���1��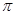 �����ɣ�����1 mol CO2 �к��еĦҼ���ĿΪ2��6.02��1023��(��2NA)��
�����ɣ�����1 mol CO2 �к��еĦҼ���ĿΪ2��6.02��1023��(��2NA)��
���㣺�����������Ų�ʽ����д�����ӿռ乹�͡��ӻ���������Լ����ۼ����йؼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ�����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ��������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��Ϫһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��12�֣�һ���ѧ�о��ɹ�������ͭ�������CuMn2O4�����ڳ����´����������е�һ����̼�ͼ�ȩ��
��1����һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������տ��Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ������������������������������
��NO3���Ŀռ乹����������������������������������������
��2����ͭ��������Ĵ��£�CO������ΪCO2��HCHO������ΪCO2��H2O��
�ٸ��ݵȵ�����ԭ����CO���ӵĽṹʽΪ��������������
��H2O������Oԭ�ӹ�����ӻ�����Ϊ��������������
��1mol CO2�к��е�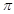 ����ĿΪ����������������������������
����ĿΪ����������������������������
��3����CuSO4��Һ�м��������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ��������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
��1����һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
��NO3���Ŀռ乹�� (����������)��
��2����ͭ��������Ĵ��£�CO��������CO2��HCHO��������CO2��H2O��
�ٸ��ݵȵ���ԭ����CO���ӵĽṹʽΪ ��
��H2O������Oԭ�ӹ�����ӻ�����Ϊ ��
��3����CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�߶�10���¿���ѧ�Ծ��������棩 ���ͣ������
(ÿ��1�� ��6��) һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1) ��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
�� Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
�� NO3���Ŀռ乹��Ϊ (����������)��
(2) ��ͭ��������Ĵ��£�CO��������CO2��HCHO��������CO2��H2O��
�� ���ݵȵ���ԭ����CO���ӵĽṹʽΪ ��
�� H2O������Oԭ�ӹ�����ӻ�����Ϊ ��
�� 1molCO2�к��еĦҼ���ĿΪ ��
(3) ��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com