
 ��һ��ɫ��Һ�����ܺ���Fe3+��Al3+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-��NO3-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��
��һ��ɫ��Һ�����ܺ���Fe3+��Al3+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-��NO3-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��| ��Ҫʵ������ | �� �� | |
| �� | ��ɫ��Ӧ������ɫ�ܲ����� | ��ɫ���� |
| �� | �ȼ���ϡ������BaCl2��Һ | ��������������dz��ְ�ɫ���� |
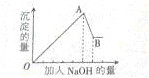
| �� | ��μ���NaOH��Һ������ | ���ɰ�ɫ�������������NaOH�����Ĺ�ϵ����ͼ��ʾ |
| �� | ��������Na2O2��ĩ | ������ɫ��ζ���壬��ɫ���� |


 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ԭ����KNO3��S |
| B������12mol����ת��ʱ����3 mol C����ԭ |
| C��1 mol S������0.5 mol C |
| D������1mol KNO3�μӷ�Ӧʱ����7.224��1024�����ӷ���ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ֻ��Na2CO3 |
| B��һ��ΪNa2CO3��NaOH |
| C��������Na2O2 |
| D��������NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| A���٢� | B���ڢ� | C���٢� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cl2ͨ���ռ���Һ�У�Cl2+2OH-�TCl-+ClO-+H2O |
| B��Al2O3��ĩ����NaOH��Һ�У�Al2O3+2OH-�T2AlO2-+H2O |
| C��AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� |
| D��FeCl2��Һ��ͨ��������Cl2��2Fe2++Cl2�T2Fe3++2Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Һ��pH=7 | ||
B�������Һ�У�c��H+��=
| ||
| C��a=b | ||
| D�������Һ�У�c��H+��+c��B+��=c��OH-��+c��A-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����һС�������ڿ����У���������NaOH |
| B��ʵ���ҿɽ��Ʊ�����ú���У�Ҳ�ɱ����������� |
| C���������Ż����ˮ���� |
| D���������ڻ�ѧ��Ӧ��ֻ������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| t/min | 0 | 2 | 5 | 10 | 15 |
| n��CO2��/mol | 1 | 0.75 | 0.5 | 0.25 | 0.25 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com