



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
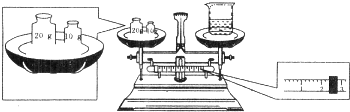
| A������12.8gCuSO4���壬��100mL�ձ��м���80mL����ˮ����ܽ� |
| B������20gCuSO4?5H2O���壬��80mL����ƿ���Ƴ���Һ |
| C��������ƿ������Һ�����У����������������ܽ������Һ������� |
| D�����ý�ͷ�ιܶ���ʱ��������ƫ�ͣ��������Ƶ���ҺŨ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| 50mL���� | 50mL���� | 50mL���� | |
| m������ | 9.2g | 15.7g | 27.6g |
| V��CO2������״���� | 2.24L | 3.36L | 3.36L |
| A����������ʵ���Ũ��Ϊ3.0mol/L |
| B����������9.2gʱ������� |
| C��15.7g�����δ��������ȫ��Ӧ |
| D�����ݱ������ݲ��ܼ�����������NaHCO3���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
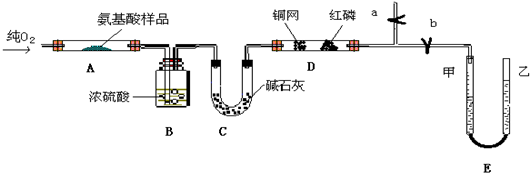
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com