
������ع㷺Ӧ���ھ�ˮ����ع�ҵ������ҵ�����Ѱ������ĸ���ƷFeSO4�Ʊ�������ص������������£�

�����ϵ�֪K2FeO4��һЩ���ʣ�
���ڼ��Ի������ȶ��������Ժ����������²��ȶ�
���ܽ�Ⱥܴ���������ˮ�Ҵ����л��ܼ�
�۾���ǿ�����ԣ��������л�����������80%�����Ҵ���Һ
�ش��������⣺
��1��д��������I���У�����Fe3+�����ӷ�Ӧ����ʽ ��
��2������II�У��Լ�AΪ (�H2O2����HNO3����NaClO��)�����˲����У��õ�������B�г�NaCl����
��3������I�а�����ȴ�ᾧ�����ˡ�ϴ�Ӹ��X�����衣ϴ�Ӹ����Ŀ�����Ѽ���ˮ�����иò���ʱ����� ϴ�ӡ�
��4����ˮʱ�������������ˮ��Ӧ������״��Fe(OH)3���벹�䲢��ƽ�÷�Ӧ����ʽ�� K2FeO4 + H2O = Fe(OH)3�� + KOH +
��5����һ������K2FeO4Ͷ��һ��Ũ�ȵ�FeCl3��Һ�У����ʣ��K2FeO4Ũ������ͼ��ʾ���Ʋ��������I������II�����ԭ���� ��

��16�֣�
��1��H2O2+2H++2Fe2+=2Fe3++2H2O��3�֣���ƽ����1�֣�
��2��NaClO Na2SO4����2�֣���4�֣�
��3����ˮ�Ҵ���3�֣�
��4��4 10 4 8 3O2 �� ��������ȷ1�֣���3�֣�
��5��FeCl3��Һˮ�������ԣ��ٽ�K2FeO4��ˮ��Ӧ���Ӷ�����K2FeO4Ũ�ȣ�3�֣�
��������
�����������1��ԭ��Ϊ����������Һ�������������Ӿ��л�ԭ�ԣ�������ǿ�ᣬ�ṩ�����ӣ������������������Ӻ������ӵ�ˮ�⣬��������ǿ�������������������ʹ������������Ϊ�����ӣ����ݻ��ϼ�������������غ㡢ԭ���غ���ƽ��������I�����������ӵ����ӷ���ʽΪH2O2+2H++2Fe2+=2Fe3++2H2O����2������Ŀ�������K2FeO4�����ڼ��Ի������ȶ��������Ժ����������²��ȶ���������II�м��������������ʹH2O2����ΪH2O2�ڼ��������µ������Ա����������������Լ�AҲ������HNO3����Ϊ����������Na2FeO4���ȶ����Լ�Aֻ���� NaClO������II�з�ӦΪ2NaOH+H2SO4=Na2SO4+2H2O��Fe2(SO4)3+3NaClO+10NaOH==2Na2FeO4+3NaCl+3Na2SO4+5H2O��������֪��Ϣ��֪��K2FeO4���ܽ�Ⱥܴ��ɴ�����Na2FeO4���ܽ�Ⱥܴ�Ϊ�˴�Na2FeO4��NaCl��Na2SO4��NaClO����������NaOH����������ɵĻ����Һ�з����Na2FeO4��NaOH���������������������������Ҫ�ɷ�ΪNaCl��Na2SO4��NaClO������������ȥ����֮�����õ�Na2FeO4��NaOH����������Һ�����Ʊ�K2FeO4����3��Na2FeO4��NaOH����������Һ�м������KOHʱ�ķ�ӦΪ��Na2FeO4+2KOH= K2FeO4+2NaOH��������Һ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӿɵ�K2FeO4���壻����K2FeO4���ܽ�Ⱥܴ���������ˮ�Ҵ����л��ܼ����Ҿ���ǿ�����ԣ��������л�����������80%�����Ҵ���Һ����ȥ���ͬʱ��Ϊ�˼���ϴ�Ӿ������K2FeO4����ʧ�����ѡ����ˮ�Ҵ�ϴ��K2FeO4���壻��4���۲�ɵã��÷�Ӧ����Ԫ����+6�۽�Ϊ+3�ۣ���Ԫ�ء���Ԫ�صĻ��ϼ۶�û�б仯������������ԭ��Ӧ�������ƶϣ����ϼ����ߵ�Ԫ��ֻ����������Ԫ���ɡ�2����Ϊ�����ڵ�0�ۣ���ȱ������Ϊ���������ݻ��ϼ���������ԭ���غ���ƽ�ɵã�4K2FeO4+10H2O==4Fe(OH)3�� +8KOH+3O2������5��������֪��Ϣ��֪��K2FeO4�ڼ��Ի������ȶ��������Ժ����������²��ȶ����Ȼ�����ǿ�������Σ���ˮ�⣬��������Һ�������ԣ�K2FeO4�����������²��ȶ�������Խǿ��K2FeO4Խ���ȶ�����Ӧ����Խ��ʣ��K2FeO4��Ũ��ԽС��
���㣺���������Ʊ���ѧ�������̣��漰���ӷ���ʽ����д����ƽ�������ɷֵ��ƶϡ�������ԭ��Ӧ����ʽ����д����ƽ�������ϴ�ӷ������Լ�����ȡ��Ϣ�����ڽ���ԭ��ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ع㷺���ڷ�ˮ�ľ�����������Ϊ������ǿ��ԭ�� | ||||||||
B������ϳ�117��Ԫ�صķ�ӦΪ��
| ||||||||
| C����ԭ�Ӿ��á��͡����ŷš�����ɫ��ѧ�ͻ����ķ�չĿ�� | ||||||||
D��������Ǽʽ����з��ֵķ����죨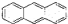 �����÷���������ԭ�ӿ��Դ���ͬһƽ�� �����÷���������ԭ�ӿ��Դ���ͬһƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ��и�����ѧ�������Ի�ѧ���� ���ͣ�ѡ����
��ѧ����ԭ�ӡ�����ˮƽ���о����ʵ���ɡ��ṹ�����ʡ��仯���Ʊ���Ӧ�õ���Ȼ��ѧ�����в����ڻ�ѧ�о���չ�����˵������ȷ���� �� ��
A��������ع㷺���ڷ�ˮ�ľ�����������Ϊ������ǿ��ԭ��
B������ϳ�117��Ԫ�صķ�ӦΪ�� ���ñ仯�����ڻ�ѧ�仯
���ñ仯�����ڻ�ѧ�仯
C����ԭ�Ӿ��á��͡����ŷš�����ɫ��ѧ�ͻ����ķ�չĿ��
D��������Ǽʽ����з��ֵķ�����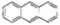 �����÷���������ԭ�ӿ��Դ���ͬһƽ��
�����÷���������ԭ�ӿ��Դ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����ԭ�ӡ�����ˮƽ���о����ʵ���ɡ��ṹ�����ʡ��仯���Ʊ���Ӧ�õ���Ȼ��ѧ�����в����ڻ�ѧ�о���չ�����˵������ȷ���� �� ��
A��������ع㷺���ڷ�ˮ�ľ�����������Ϊ������ǿ��ԭ��
B������ϳ�117��Ԫ�صķ�ӦΪ��![]() ���ñ仯�����ڻ�ѧ�仯
���ñ仯�����ڻ�ѧ�仯
C����ԭ�Ӿ��á��͡����ŷš�����ɫ��ѧ�ͻ����ķ�չĿ��
D��������Ǽʽ����з��ֵķ�����![]() �����÷���������ԭ�ӿ��Դ���ͬһƽ��
�����÷���������ԭ�ӿ��Դ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����ԭ�ӡ�����ˮƽ���о����ʵ���ɡ��ṹ�����ʡ��仯���Ʊ���Ӧ�õ���Ȼ��ѧ�����в����ڻ�ѧ�о���չ�����˵������ȷ���� �� ��
A��������ع㷺���ڷ�ˮ�ľ�����������Ϊ������ǿ��ԭ��
B������ϳ�117��Ԫ�صķ�ӦΪ��![]() ���ñ仯�����ڻ�ѧ�仯
���ñ仯�����ڻ�ѧ�仯
C����ԭ�Ӿ��á��͡����ŷš�����ɫ��ѧ�ͻ����ķ�չĿ��
D��������Ǽʽ����з��ֵķ����������÷���������ԭ�ӿ��Դ���ͬһƽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com