

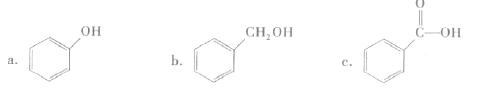




| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΈ ¥πΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΧνΩ’Χβ
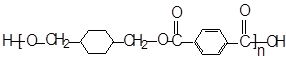
 Θ≠CH3+Cl2Γζ
Θ≠CH3+Cl2Γζ Θ≠CH2Cl+HCl
Θ≠CH2Cl+HCl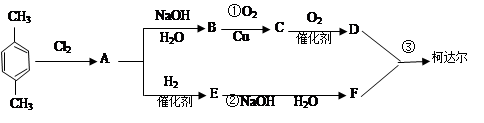
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
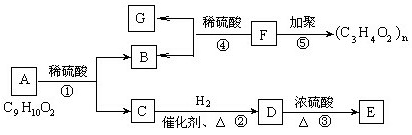
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΒΞ―ΓΧβ
| AΘ°÷Μ”–ΔΌ | BΘ°÷Μ”–ΔέΔήΔί | CΘ°÷Μ”–ΔΌΔΎΔέΔί | DΘ°ΕΦ≤Μ–– |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΧνΩ’Χβ
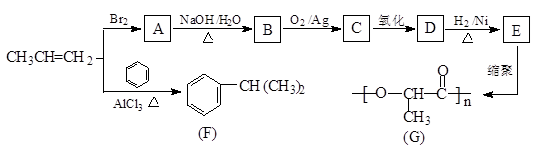
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΧνΩ’Χβ
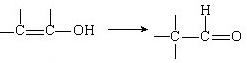
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
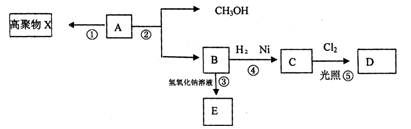
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΒΞ―ΓΧβ
| AΘ°¬χΆΖΓΔΟΉΖΙ‘ΎΩΎ«ΜΡΎ‘ΫΫά‘ΫΧπΘ§ «“ρΈΣΥϋΟ«Κ§”–ΒΡΒμΖέΖΔ…ζΝΥθΞΜ·Ζ¥”Π |
| BΘ°Χ«άύΓΔ”Ά÷§ΓΔΒΑΑΉ÷ ΕΦΡήΖΔ…ζΥ°ΫβΖ¥”Π |
| CΘ°≤ΥΉ―”ΆΓΔ≈Θ”ΆΓΔΜ®…ζ”ΆΕΦ τ”ΎθΞάύ |
| DΘ°ΤœΧ―Χ«Ζ÷Ή”ΫαΙΙ÷–÷Μ”–“ΜΗω»©ΜυΘ§Υυ“‘ «ΒΞΧ« |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΧνΩ’Χβ
 Ϋ ΘΜ
Ϋ ΘΜ≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ≤Μœξ Χβ–ΆΘΚΒΞ―ΓΧβ
AΘ°CH3N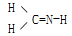 | BΘ°CH4Si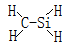 |
CΘ°CH2SO 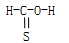 | DΘ°CH4S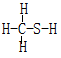 |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com