
| 1 | 4 |
| c(H2S) |
| c(H2) |
| c(H2S) |
| c(H2) |
| 3mol |
| 3mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ��и�����һ������Կ������ۻ�ѧ�Ծ��������棩 ���ͣ������
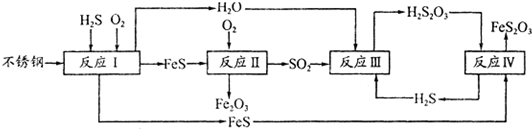
�й����غϳ���(����)ʹ��������Ϊŷ�����ҵ�1/4��Ϊ�˱��������о� Ժ���Ĵ����컯������ʴ���̽����о����ó����и�ʴ������

(1)H2S���Ժϳ����ص���Ȼ������380 K�����Ϊ2 L���ܱ������У��������·�Ӧ��H2(g)��S(s) H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2 S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��
H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2 S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��

(2)�ڷ�ӦI�з����Ļ�ѧ��ӦΪ______��
(3)�о����ַ�ӦII�Ƿֱ���Fe��FeSΪ�缫����ˮĤΪ�������Һ�ĵ绯ѧ��ʴ���� ��
��Ϊ______��
��֪��Fe(s)��S(s)=FeS(s) ��H1=��2��5akJ��mol��1
S(s)��O2(g)=SO2(g) ��H2=��5akJ��mol��1
4Fe(s)��3O2(g)=2Fe2O3(s) ��H3=��6akJ��mol��1
��ӦII���Ȼ�ѧ����ʽΪ_____
(4)��֪H2S2O3��K1=2��2��10-1��K2=2��8��10-2��Na2S2O3ˮ��Һ��______�ԣ��� ��Һ�е���غ�ʽΪ_____ ����ӦIY�ķ�Ӧ����Ϊ______ ���÷�Ӧ______(��ܡ����ܡ��� ˵��FeS�ܽ���ǿ��FeS2O3
(5)���컯���������ո�ʴ����Ϊ______��Ϊ����Ч���������ֽ������컯���������� CuSO4��Һ������(H2S)���������漰�����ӷ���ʽΪ
__________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com