
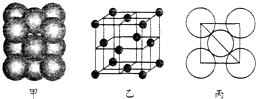
·ÖĪö £Ø1£©øł¾ŻŌŖĖŲŹŲŗćÖŖ£¬AÖŠŗ¬ÓŠNa”¢CŌŖĖŲ£¬øł¾ŻŌ×ÓŹŲŗćÖŖ£¬»¹ŗ¬ÓŠOŌŖĖŲ£¬ĒŅNa”¢C”¢OŌ×ÓøöŹż·Ö±šŹĒ2”¢1”¢3£¬ĖłŅŌAĪŖNa2CO3£»ÓÉŅõŃōĄė×Ó¹¹³ÉµÄ¾§ĢåĪŖĄė×Ó¾§Ģ壻
£Ø2£©ÕżøŗµēŗÉÖŠŠÄ²»ÖŲŗĻµÄ·Ö×ÓĪŖ¼«ŠŌ·Ö×Ó£»øł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪ¼ĘĖć²¢ÅŠ¶ĻÖŠŠÄŌ×ÓµÄŌӻƹģµĄĄąŠĶŗĶĪ¢Į£µÄæռ乹ŠĶ£»
£Ø3£©æÉŅŌ¾łĢƷؼĘĖć¾§°ūÖŠAlŌ×ÓŹżÄ棻½įŗĻAlµÄĦ¶ūÖŹĮæ¼ĘĖć¾§°ūÖŹĮ棬AlµÄŌ×Ó°ė¾¶ĪŖd pm£¬Ōņ¾§°ūĄā³¤ĪŖ4d pm”Į$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$d pm£¬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖć¾§°ūĆÜ¶Č£®
½ā“š ½ā£ŗ£Ø1£©øł¾ŻŌŖĖŲŹŲŗćÖŖ£¬AÖŠŗ¬ÓŠNa”¢CŌŖĖŲ£¬øł¾ŻŌ×ÓŹŲŗćÖŖ£¬»¹ŗ¬ÓŠOŌŖĖŲ£¬ĒŅNa”¢C”¢OŌ×ÓøöŹż·Ö±šŹĒ2”¢1”¢3£¬ĖłŅŌAĪŖNa2CO3£»ÓÉŅõŃōĄė×Ó¹¹³ÉµÄ¾§ĢåĪŖĄė×Ó¾§Ģ壬Ģ¼ĖįÄĘŹĒÓÉÄĘĄė×ÓŗĶĢ¼ĖįøłĄė×Ó¹¹³ÉµÄ£¬ĪŖĄė×Ó¾§Ģ壬
¹Ź“š°øĪŖ£ŗĄė×Ó£»
£Ø2£©·Ö×Ó¾§ĢåÓÉ·Ö×Ó¹¹³É£¬ÕżøŗµēŗÉÖŠŠÄ²»ÖŲŗĻµÄ·Ö×ÓĪŖ¼«ŠŌ·Ö×Ó£¬¶žŃõ»ÆĢ¼ŗĶH2O¶¼ŹĒ·Ö×Ó¾§Ģ壬¶žŃõ»ÆĢ¼ÖŠÕżøŗµēŗÉÖŠŠÄÖŲŗĻĪŖ·Ē¼«ŠŌ·Ö×Ó£¬H2OÖŠÕżøŗµēŗÉÖŠŠÄ²»ÖŲŗĻ£¬ĪŖ¼«ŠŌ·Ö×Ó£»Ė®ÖŠŠÄŌ×ÓOµÄ¼Ū²ćµē×Ó¶ŌŹżĪŖ4£¬ÓŠĮ½¶Ō¹Ā¶Ōµē×Ó¶Ō£¬¹ģµĄŌӻƷ½Ź½ĪŖsp3£¬·Ö×Ó¹¹ŠĶŹĒCŠĪ£¬
¹Ź“š°øĪŖ£ŗH2O£»sp3£»VŠĪ£»
£Ø3£©Ōņ¾§°ūÖŠĆæøöA1Ō×ÓÖÜĪ§ÓėĖü×ī½Ó½üµÄĒŅ¾ąĄėĻąµČµÄA1Ō×ÓµÄøöŹżĪŖÉĻĆę6øÉøö£¬ĻĀĆę6øö£¬¹²12øö£¬¾§°ūÖŠAlŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+6”Į$\frac{1}{2}$=4£¬¾§°ūÖŹĮæĪŖ4”Į$\frac{27}{{N}_{A}}$g£¬AlµÄŌ×Ó°ė¾¶ĪŖd pm£¬Ōņ¾§°ūĄā³¤ĪŖ4d pm”Į$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$d pm£¬¹ŹAl¾§°ūµÄĆܶȦŃ=$\frac{m}{V}$=4”Į$\frac{27}{{N}_{A}}$g”Ā£Ø2$\sqrt{2}$d”Į10-10cm£©3=$\frac{M}{4\sqrt{2}{{d}^{3}N}_{A}}$g£®cm-3£¬
¹Ź“š°øĪŖ£ŗ12£»4£»$\frac{M}{4\sqrt{2}{{d}^{3}N}_{A}}$g£®cm-3£®
µćĘĄ ±¾Ģā漲龧Ģå½į¹¹”¢¾§°ū¼ĘĖćµČÖŖŹ¶µć£¬ĪŖøßæ¼øßʵµć£¬×¢ŅāĄūÓĆ¾łĢƷؼĘĖć¾§°ūÖŹĮ棬ŠčŅŖѧɜ¾ßÓŠŅ»¶ØµÄŹżŃ§¼ĘĖćÄÜĮ¦£¬ÄѶČÖŠµČ£®


 ¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²Ėµ¶ĒŠĻĢČāŗóĪ““¦Ąķ¶ųÉśŠā | B£® | ÓĆŹ³“׳żČ„Ė®ŗųÖŠµÄĖ®¹ø | ||
| C£® | ÓĆĘĻĢŃ×ŌÄšĘĻĢŃ¾Ę | D£® | ½«»Ę¹ĻĒŠ³Éʬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
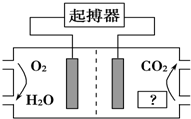 ČēĶ¼ĖłŹ¾£¬×ī½üĆĄ¹śŅ½Ń§¼ŅĄūÓĆČĖĢå×ŌÉķ»·¾³Éč¼ĘĮĖŠÄŌąĘš²«Ę÷£¬Ęä¶ÆĮ¦ÓÉČĖĢåĢåŅŗÖŠµÄÄÜĮæĪļÖŹĢį¹©£®ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ČēĶ¼ĖłŹ¾£¬×ī½üĆĄ¹śŅ½Ń§¼ŅĄūÓĆČĖĢå×ŌÉķ»·¾³Éč¼ĘĮĖŠÄŌąĘš²«Ę÷£¬Ęä¶ÆĮ¦ÓÉČĖĢåĢåŅŗÖŠµÄÄÜĮæĪļÖŹĢį¹©£®ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | Ķ¼ÖŠ”°£æ”±ĪŖČĖĢåÖŠµÄÖ¬·¾ | |
| B£® | O2¼«ĪŖŠÄŌąĘš²«Ę÷µÄøŗ¼« | |
| C£® | ²śÉśµÄCO2Ź¹ČĖĢåŃŖŅŗ³ŹĖįŠŌ£¬³¤ĘŚŹ¹ÓƶŌČĖĢåÓŠŗ¦ | |
| D£® | µē³ŲÕż¼«µÄµē¼«·“Ó¦Ź½ĪŖO2+4H++4e-ØT2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
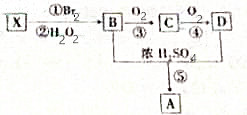 XŹĒŅ»ÖÖ³£¼ūĘųĢ¬Ģž£¬ĖüæÉÓÉŅŅ“¼ÓėÅØĮņĖį¼ÓČȵ½170”ęÖĘµĆ£®ĻÖŅŌXĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅ»ÖÖ¾ßÓŠ¹ūĻćĪ¶µÄ»·×“ĪļÖŹA£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£®ĘäÖŠ¢Ū”¢¢ÜµÄ“߻ƼĮµČĢõ¼ž²»Ķ¬£»ÓÖŅŃÖŖRBrŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³ÉROH£®
XŹĒŅ»ÖÖ³£¼ūĘųĢ¬Ģž£¬ĖüæÉÓÉŅŅ“¼ÓėÅØĮņĖį¼ÓČȵ½170”ęÖĘµĆ£®ĻÖŅŌXĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅ»ÖÖ¾ßÓŠ¹ūĻćĪ¶µÄ»·×“ĪļÖŹA£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£®ĘäÖŠ¢Ū”¢¢ÜµÄ“߻ƼĮµČĢõ¼ž²»Ķ¬£»ÓÖŅŃÖŖRBrŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³ÉROH£® +2H2O£®
+2H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO2ŌŚ“Ė¹ż³ĢÖŠ×÷Ńõ»Æ¼Į | |
| B£® | ³¬ĻøĶ·ŪÄܵ¼µē£¬ĖłŅŌ³¬ĻøĶ·ŪŹĒµē½āÖŹ | |
| C£® | ¹¤ŅµÉĻ³£ÓƵē½ā·ØŅ±Į¶Ķ | |
| D£® | ĄķĀŪÉĻÖʵĆ1molĶ·Ū£¬¹²×ŖŅĘ3mole- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
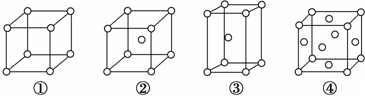
| A£® | ¢ŚĪŖĮł·½×īĆܶѻż£¬¢ŪĪŖĢåŠÄĮ¢·½¶Ń»ż | |
| B£® | ¢ŪŗĶ¢ÜµÄÅäĪ»Źż¶¼ŹĒ12 | |
| C£® | ĶźČ«ŹōÓŚ¢ÜµÄ½šŹōŌ×ÓŹżĪŖ6øö | |
| D£® | ¢ŪµÄæÕ¼äĄūÓĆĀŹŠ”ÓŚ¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
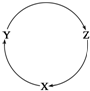 ĻĀĮŠø÷×éĪļÖŹÖŠ£¬²»ÄÜĀś×ćĻĀĶ¼ĪļÖŹŅ»²½×Ŗ»Æ¹ŲĻµµÄŃ”ĻīŹĒ£Ø””””£©
ĻĀĮŠø÷×éĪļÖŹÖŠ£¬²»ÄÜĀś×ćĻĀĶ¼ĪļÖŹŅ»²½×Ŗ»Æ¹ŲĻµµÄŃ”ĻīŹĒ£Ø””””£©| Ń”Ļī | X | Y | Z |
| A | NaOH | Na2CO3 | NaHCO3 |
| B | C | CO | CO2 |
| C | Cu | CuSO4 | Cu£ØOH£©2 |
| D | AlCl3 | NaAlO2 | Al£ØOH£©3 |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com