
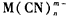 ����ʽ������ˮ�С��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�
����ʽ������ˮ�С��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�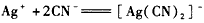 �յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ����������֪ʶ�ش��������⣺
�յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ����������֪ʶ�ش��������⣺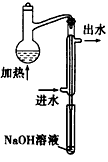
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ɡ�ұ���͵�ƹ�ҵ������������軯�������ˮ�����к��軯������HCN��CN-�ͽ������ӵ�������M��CN��nm-����ʽ������ˮ�У��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�
��ɡ�ұ���͵�ƹ�ҵ������������軯�������ˮ�����к��軯������HCN��CN-�ͽ������ӵ�������M��CN��nm-����ʽ������ˮ�У��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�꽭�վ���(10��)��ɡ�ұ���͵�ƹ�ҵ������������軯�������ˮ�����к��軯������HCN��CN -�ͽ������ӵ�������M(CN)nm-����ʽ������ˮ�С��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�
��ˮ��Ԥ������ˮ���м��������EDTA����pH��2�������¼��������������е�HCN������NaOH��Һ���ա�
�ڵζ���������Һ������pH��11������������ָʾ������AgNO3����Һ�ζ�
 Ag++2CN - == [Ag(CN)2] -
Ag++2CN - == [Ag(CN)2] -
�յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ��
��������֪ʶ�ش��������⣺
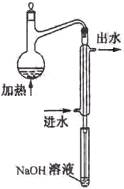
��ˮ��Ԥ������Ŀ���� ��
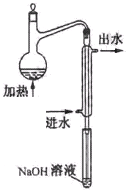
��ˮ��Ԥ������װ������ͼ��ϸ���ܲ�������Һ����Ϊ�� ____
������ƿ������Һ��Ҫ�߳��ܶ࣬��Ŀ���� ��
���������������������Ԥ������ʵ������ (�ƫ�ߡ�������Ӱ�족��ƫ�͡�)��
��ȷ��ȡij������ˮ100mL������������Ũ��Ϊ0.01000mol?L-1������������Һ�ζ����յ�ʱ������21.00mL����ˮ���к��軯����ĺ���Ϊ mg?L-1��(�Լƣ�����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ��Ԥ������ˮ���м��������EDTA����pH��2�������¼��������������е�HCN������NaOH��Һ���ա�
�ڵζ���������Һ������pH��11������������ָʾ������AgNO3����Һ�ζ�
Ag++2CN - = [Ag(CN)2] -
�յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ��
��������֪ʶ�ش��������⣺
��ˮ��Ԥ������Ŀ���� ��
��ˮ��Ԥ������װ������ͼ��ϸ���ܲ�������Һ����Ϊ�� ��
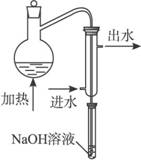
������ƿ������Һ��Ҫ�߳��ܶ࣬��Ŀ���� ��
���������������������Ԥ������ʵ������ (�ƫ�ߡ�������Ӱ�족��ƫ�͡�)��
��ȷ��ȡij������ˮ100mL������������Ũ��Ϊ0.01000mol��L-1������������Һ�ζ����յ�ʱ������21.00mL����ˮ���к��軯����ĺ���Ϊ mg��L-1��(��CN-�ƣ�����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɡ�ұ���͵�ƹ�ҵ������������軯�������ˮ�����к��軯������HCN��CN -�ͽ������ӵ�������M(CN)nm-����ʽ������ˮ�С��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�
��ˮ��Ԥ������ˮ���м��������EDTA����pH��2�������¼��������������е�HCN������NaOH��Һ���ա�
�ڵζ���������Һ������pH��11������������ָʾ������AgNO3����Һ�ζ�
Ag++2CN - == [Ag(CN)2] -
�յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ��
��������֪ʶ�ش��������⣺
��1��ˮ��Ԥ������Ŀ���� ��
��2��ˮ��Ԥ������װ������ͼ��ϸ���ܲ�������Һ����Ϊ�� ��
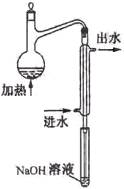
��3������ƿ������Һ��Ҫ�߳��ܶ࣬��Ŀ���� ��
��4���������������������Ԥ������ʵ������ (�ƫ�ߡ�������Ӱ�족��ƫ�͡�)��
��5��ȷ��ȡij������ˮ100mL������������Ũ��Ϊ0.01000mol��L-1������������Һ�ζ����յ�ʱ������21.00mL����ˮ���к��軯����ĺ���Ϊ mg��L-1����CN-�ƣ�����������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ���⣨���վ��� ���ͣ�ʵ����
(10��)��ɡ�ұ���͵�ƹ�ҵ������������軯�������ˮ�����к��軯������HCN��CN -�ͽ������ӵ�������M(CN)nm-����ʽ������ˮ�С��ⶨ��ˮ�к��軯���ﺬ����ʵ�鲽�����£�
��ˮ��Ԥ������ˮ���м��������EDTA����pH��2�������¼��������������е�HCN������NaOH��Һ���ա�
�ڵζ���������Һ������pH��11������������ָʾ������AgNO3����Һ�ζ�
Ag++2CN - ="=" [Ag(CN)2] -
�յ�ʱ����Һ�ɻ�ɫ��ɳȺ�ɫ��
��������֪ʶ�ش��������⣺
��1��ˮ��Ԥ������Ŀ���� ��
��2��ˮ��Ԥ������װ������ͼ��ϸ���ܲ�������Һ����Ϊ�� ��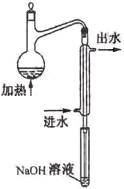
��3������ƿ������Һ��Ҫ�߳��ܶ࣬��Ŀ���� ��
��4���������������������Ԥ������ʵ������ (�ƫ�ߡ�������Ӱ�족��ƫ�͡�)��
��5��ȷ��ȡij������ˮ100mL������������Ũ��Ϊ0.01000mol��L-1������������Һ�ζ����յ�ʱ������21.00mL����ˮ���к��軯����ĺ���Ϊ mg��L-1����CN-�ƣ�����������һλС������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com