
(2)Ņ»ĘæĪŽÉ«ĘųĢ壬æÉÄÜŗ¬ÓŠCH4ŗĶCH2=CH2»ņĘäÖŠµÄŅ»ÖÖ£¬ÓėŅ»ĘæCl2»ģŗĻŗó¹āÕÕ£¬¹Ū²ģµ½»ĘĀĢÉ«Öš½„ĶŹČ„£¬Ęæ±ŚÓŠÉŁĮæĪŽÉ«ÓĶדŠ”ŅŗµĪ”£
¢ŁÓÉÉĻŹöŹµŃéĻÖĻóĶʶĻ³öøĆĘæĘųĢåÖŠŅ»¶Øŗ¬ÓŠCH4£¬ÄćČĻĪŖŹĒ·ńÕżČ·£¬ĪŖŹ²Ć“£æ
______________________________________ӣ
¢ŚÉĻŹöŹµŃéæÉÄÜÉę¼°µÄ·“Ó¦ĄąŠĶÓŠ__________________________”£
(3)ŗ¬ÓŠ![]() µÄ»ÆŗĻĪļÓėCH2=CH2Ņ»Ńł£¬ŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻ³Éøß·Ö×Ó»ÆŗĻĪļ”£
µÄ»ÆŗĻĪļÓėCH2=CH2Ņ»Ńł£¬ŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻ³Éøß·Ö×Ó»ÆŗĻĪļ”£
¢Ł¹ć·ŗÓĆ×÷Å©ÓƱ”ĤµÄ¾ŪĀČŅŅĻ©ĖÜĮĻ£¬ŹĒÓÉ![]() ¾ŪŗĻ¶ų³ÉµÄ£¬Ęä»Æѧ·½³ĢŹ½ŹĒ____________________________________________”£
¾ŪŗĻ¶ų³ÉµÄ£¬Ęä»Æѧ·½³ĢŹ½ŹĒ____________________________________________”£
¢ŚµēĘ÷°ü×°ÖŠ“óĮæŹ¹ÓƵÄÅŻÄĖÜĮĻµÄÖ÷ŅŖ³É·ÖŹĒ¾Ū±½ŅŅĻ©(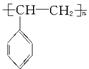 )£¬ĖüŹĒÓÉ_____________(Š“½į¹¹¼ņŹ½)¾ŪŗĻ¶ų³ÉµÄ”£
)£¬ĖüŹĒÓÉ_____________(Š“½į¹¹¼ņŹ½)¾ŪŗĻ¶ų³ÉµÄ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ





²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢æÉÓĆĖ®¼ų±š±½”¢ĖÄĀČ»ÆĢ¼”¢ŅŅ“¼ČżÖÖĪŽÉ«ŅŗĢå | B”¢Č”1 gNaOH¹ĢĢåÓŚÉÕ±£¬¼ÓČė9 mLĖ®£Ø¦Ń=1g?cm-3£©³ä·Ö½Į°čÅä³É10%µÄNaOHČÜŅŗ | C”¢ÓĆNaOHČÜŅŗ¾Ķæɼų±šK+”¢Mg2+”¢Al3+”¢Fe2+”¢Fe3+ĪåÖÖĄė×Ó | D”¢ĪŖ×¼Č·²ā¶ØŃĪĖįÓėNaOHČÜŅŗ·“Ó¦µÄÖŠŗĶČČ£¬ĖłÓĆĖįŗĶ¼īµÄĪļÖŹµÄĮæÓ¦ĻąµČ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com