
(1)X�����к��й����ŵ����ƣ�________________��
(2)X���ӵĽṹ��ʽΪ��______________________��
(3)X������һ�������¿ɷ������ֻ�ѧ��Ӧ(��ͼ)��
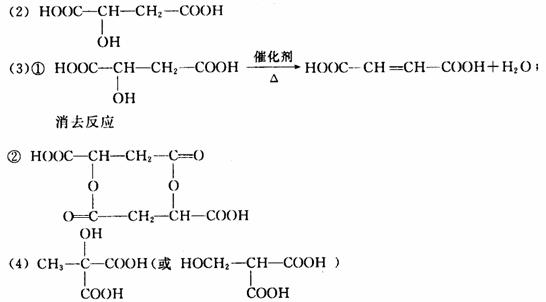
��д����Ӧ�ٵĻ�ѧ����ʽ��________________���䷴Ӧ����Ϊ��________________��
��д��Z�Ľṹ��ʽ��___________________��
(4)X��ͬ���칹���ж��֣���д����Xͬ����һ��ͬ���칹��Ľṹ��ʽ________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
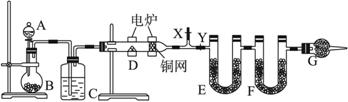
����������⣺
(1)��ƿB��Ϊ��ɫ���壬�����з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
(2)ͭ����������________________________��
(3)F�е��Լ������____________��
(4)װ��G��������________________________��
(5)�ڿ�ʼ����D֮ǰ���ȴ���X���رռ���Y��ͨһ��ʱ���������ٹرռ���X������Y��Ȼ��ʼ���ȣ�����������Ŀ����______________________________________��
(6)��ȡ1.80 gij�л������(�������ĺ���������)�������������ⶨ��E������10.8 g��F������2.64 g������л�������ʽΪ_________________________�����Ҫȷ���л���ķ���ʽ������ⶨ��������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ��Ԫ�и����ڶ�������Կ������ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
������A������ʽΪC3H10;ij����������X������ʽΪC15H14O3����ʹFeC13��Һ����ɫ��J�����ں˴Ź�����������4���������շ塣��һ�������������µ�ת����ϵ��
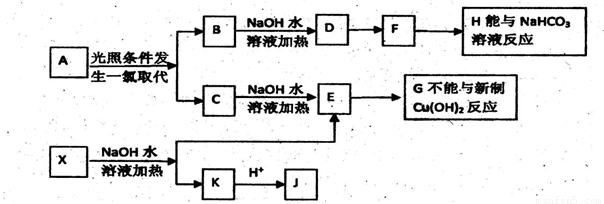
��1��A�Ľṹ��ʽΪ____��X�Ľṹ��ʽΪ ��
��2��J�������ĺ��������ŵ�����Ϊ___ _��
��3��E��H��Ӧ�Ļ�ѧ����ʽ��____ ��
��4����������Һ�е���F������Ӧ�Ļ�ѧ����ʽΪ___ _��
��5��B��C�Ļ������NaOH�Ҵ���Һ�м��ȿ�������ͬһ���л���I����IΪ����ϳɵĸ߷��ӻ�����Ľṹ��ʽ�� ��
��6����֪J�ж���ͬ���칹�壬��д����������������J��ͬ���칹��Ľṹ��ʽ ������FeCl3��Һ��������ɫ���ڿ��Է���������Ӧ���۱�����ֻ��2�ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ĩ�� ���ͣ������
ͨ����ʳ���Ϳɻ��ij���ĺ���������X������Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13.0%��
��1��X�ķ���ʽ��____________��
��2������ʳ���ͻ��X�Ļ�ѧ�仯�����У�����Ҫ��������ѧ����ʽ��________________________��
________________________��
��3��X������Ʒ�Ӧ�ų���������Ӧ�Ļ�ѧ����ʽ��_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com