��2013?���϶�ģ�����Na
2CO
3�������������о��й㷺����;��ͼ1��ʵ����ģ���Ƽ�ԭ����ȡNa
2CO
3������ͼ��
��֪����ʳ��ˮ��ͨ��NH
3��CO
2�����ͷ�ӦΪNaCl+NH
3+CO
2+H
2O��NaHCO
3��+NH
4Cl����ش��������⣺
��1������������������Ca
2+��Mg
2+��SO
42-�ȣ�
���Ƴ��ӵIJ���˳��a��
c
c
��
d
d
��
e
e
��b������ĸ��ţ���
a�������ܽ⣬��ȥ������b�����������pH��c������Ba��OH��
2��Һ��d������Na
2CO
3��Һ��e������
��ʳ��ˮ����ͨ��NH
3����ͨ��CO
2��������
NH3������ˮ�������������ܽ�Ȳ����CO2
NH3������ˮ�������������ܽ�Ȳ����CO2
��
��2�����չ���A��Na
2CO
3��
a
a
������ĸ��ţ��н��У�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH
4+�ķ�����
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+
��
����ҺA�����ؽᾧ�ܹ����NH
4HCO
3����pH=13��Na
+��K
+����Һ�м�������NH
4HCO
3��ʹpH���ͣ���Ӧ�����ӷ���ʽ
NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O
NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O
��
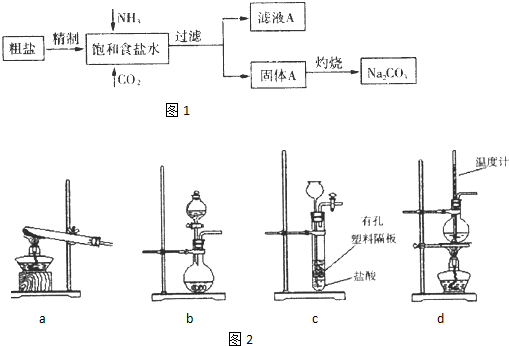
��3��ͼ2װ���г�����ʵ�����Ʊ�CO
2����
bc
bc
������ĸ��ţ�����bʾ���װ���Ʊ�NH
3����Һ©����ʢ�ŵ��Լ�
Ũ��ˮ
Ũ��ˮ
�����Լ����ƣ�����ƿ�ڿɼ���Ĺ����Լ�
��ʯ�ң���NaOH���壩
��ʯ�ң���NaOH���壩
�����Լ����ƣ���
��4��һ����Ȼ���ɷ���aNa
2CO
3?bNa
2CO
3?cH
2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa
2CO
3������������ʵ����������������ѡ�����ʵ�鷽����ȫ����ѡ����Լ���1mol?L
-1H
2SO
4��Һ��1.0mol?L
-1BaCl
2��Һ��ϡ��ˮ����ʯ�ҡ�Ca��OH��
2��Һ������ˮ��
�ٳ�ȡm
1gһ������Ȼ�����Ʒ��������������ˮ�У�
��
��������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ
��������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ
��
��
���ˡ�ϴ�ӡ������������
���ˡ�ϴ�ӡ������������
��
�ܼ�����Ȼ����к�Na
2CO
3������������




 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�