
ij������ȫ���ҵIJ���ҩ����Ҫ����NaN3��Fe2O3��KClO4��NaHCO3�����ʡ�������������ײʱ������ҩ��������������ʹ����Ѹ�����ͣ��Ӷ��������á�
��1��NaN3�����巢���������ȷֽ����N2��Na��N2�ĵ���ʽΪ________��
��2��Fe2O3��������������Na�����û���Ӧ���ɵĻ�ԭ����Ϊ________��
��3��KClO4��������������Ӧ��������Na��������KCl��Na2O��KClO4���л�ѧ��������Ϊ________����������K�Ķѻ���ʽΪ________��
��4��NaHCO3����ȴ�������ղ����������ͷŵ������������ֽ⣬�仯ѧ����ʽΪ______
__________________________________��
��5��100 g��������ҩ������������ͨ����ʯ�Һ�õ�N2 33.6 L����״������
���ü�ʯ�ҳ�ȥ������Ϊ________________��
�ڸò���ҩ����NaN3����������Ϊ________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016���������ʦ���и����µ����������ƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й���Һ������Ũ�ȵĹ�ϵʽ�У���ȷ���ǣ� ��
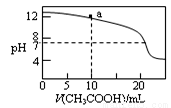
A��pH��ͬ�Ģ�CH3COONa����NaHCO3������Һ�е�c(Na��)���ڣ���
B��0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У�c(Na+)=2c(A2-)��c(HA-)��c(H2A)
C����ͼ��ʾ��0.1 mol/L CH3COOH��Һ�ζ�20 mL 0.1mol/L NaOH��Һ�ĵζ����ߣ���pH��7ʱ��c(Na��)��c(CH3COO��) ��c(OH��)��c(H��)
D����ͼa����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c(OH��)��c(H��)��c(CH3COO��)��2c(CH3COOH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�걱���к��Ϸ�У��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���Ѵﵽƽ��Ŀ��淴Ӧ2SO2+O2 2SO3�У�������
2SO3�У�������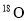 ��ɵ�����һ��ʱ���
��ɵ�����һ��ʱ��� ���������������е�( )
���������������е�( )
A�������������
B�����ɵ�����������
C�������Ͷ���������
D��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ۡ������ʡ�������ˮ��Һ�����οɷֱ�ʹ�õ��Լ��Ͷ�Ӧ��������ȷ���ǣ� ��
A���� ˮ������ɫ ������Cu(OH)2��ש��ɫ������Ũ���ᣬ���ɫ
ˮ������ɫ ������Cu(OH)2��ש��ɫ������Ũ���ᣬ���ɫ
B��Ũ���ᣬ���ɫ ������Cu(OH)2��ש��ɫ��������ˮ������ɫ
C������Cu(OH)2��ש��ɫ��������ˮ������ɫ��Ũ���ᣬ���ɫ
D����ˮ������ɫ �� Ũ���ᣬ���ɫ ������Cu(OH)2��ש��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�йػ�ѧ������ȷ���ǣ� ��
A����ϩ�Ľṹ��ʽ��CH2CH2
B���������Ľṹ��ʽ��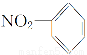
C�����Ȼ�̼�ĵ���ʽ: 
D��������ӵı���ģ��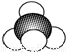
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶��µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ж�һЩʵ����ʵ�����۽�����ȷ���� �� ��
ѡ�� | ʵ����ʵ | ���۽��� |
A | ��ԭ�ӵĵ�һ�����ܴ�����ԭ�� | ��ԭ��2p�������� |
B | CO2Ϊֱ���η��� | CO2������C |
C | ���ʯ���۵����ʯī | ���ʯ�Ƿ��Ӿ��壬ʯī��ԭ�Ӿ��� |
D | HF�ķе����HCl | HF����Է�������С��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶��µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ��������ȷ���ǣ� ��
A����1 mol��L��1 AlCl3��Һ�мӹ�����6 mol��L��1 NaOH��Һ���Ʊ�Al��OH��3����Һ
B������ͨ����ˮCuSO4����ĩ������֤��ԭ�����к���ˮ����
C�����հ�ɫ��ĩ������ʻ�ɫ��֤��ԭ��ĩ����Na������K��
D����������Fe3����CuSO4��Һ�м���ͭƬ�ɳ�ȥFe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶�5���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
1molij������2molHCl��ȫ�ӳɣ����������ܱ�8molCl2��ȫȡ������ԭ��������Ϊ�� ��
A��1,3-����ϩ B��������ϩ C��2-��Ȳ D��1-��Ȳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��������������ѧУ�߶��µڶ��ο���ѧ�Ծ��������棩 ���ͣ�ѡ����
�л�������A(C8H8O2)Ϊһ����ɫҺ�塣��A�����ɷ�����ͼ��һϵ�з�Ӧ��������˵����ȷ���ǣ� ��

A���������������ܷ���ˮ�ⷴӦ����A��B��D��G
B������ͼʾ����֪DΪ����
C��A�Ľṹ�к���̼̼˫��
D��G��ͬ���칹�������������ܷ���������Ӧ��ֻ��һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com