
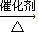
 2CH3COOH£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
2CH3COOH£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ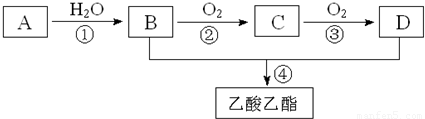
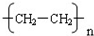 £¬øĆ·“Ó¦ŹōÓŚ¼Ó¾Ū·“Ó¦£¬
£¬øĆ·“Ó¦ŹōÓŚ¼Ó¾Ū·“Ó¦£¬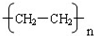 £¬¼Ó¾Ū·“Ó¦£»
£¬¼Ó¾Ū·“Ó¦£»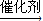 CH3CH2OH
CH3CH2OH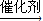 CH3CH2OH£»
CH3CH2OH£»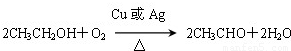 £¬
£¬ £»
£»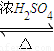 CH3COOCH2CH3+H2O£¬
CH3COOCH2CH3+H2O£¬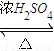 CH3COOCH2CH3+H2O£®
CH3COOCH2CH3+H2O£®

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĆĄ¹śĀŽŹĻÉś²śµÄ°ĀĖ¾ĖūĪ¤Į×ĖįŃĪ½ŗÄŅ¼Į£ØÉĢĘ·ĆūÖŠ¹ś“óĀ½³Ę“ļ·Ę?øŪŅėĢŲĆōø££¬ĢØĶåŅėĪŖæĖĮ÷øŠ£©ŹĒŹŠ³”ÉĻĪØŅ»µÄ°ĀĖ¾ĖūĪ¤ÖĘ¼Į£®2009ÄźÓÉÓŚ¼×ŠĶH1N1ŌŚŹĄ½ē·¶Ī§µÄĄ©É¢£¬Č«ĒņĻĘĘšŅ»¹ÉĒĄ¹ŗ“ļ·Ę·ē³±£®°ĀĖ¾ĖūĪ¤µÄ½į¹¹ČēĶ¼£»ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ĆĄ¹śĀŽŹĻÉś²śµÄ°ĀĖ¾ĖūĪ¤Į×ĖįŃĪ½ŗÄŅ¼Į£ØÉĢĘ·ĆūÖŠ¹ś“óĀ½³Ę“ļ·Ę?øŪŅėĢŲĆōø££¬ĢØĶåŅėĪŖæĖĮ÷øŠ£©ŹĒŹŠ³”ÉĻĪØŅ»µÄ°ĀĖ¾ĖūĪ¤ÖĘ¼Į£®2009ÄźÓÉÓŚ¼×ŠĶH1N1ŌŚŹĄ½ē·¶Ī§µÄĄ©É¢£¬Č«ĒņĻĘĘšŅ»¹ÉĒĄ¹ŗ“ļ·Ę·ē³±£®°ĀĖ¾ĖūĪ¤µÄ½į¹¹ČēĶ¼£»ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ”÷ |



| “߻ƼĮ |
| “߻ƼĮ |


| ÅØH2SO4 |
| ”÷ |
| ÅØH2SO4 |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢NH4HC03 | B”¢K2S04 | C”¢Ca3£ØP04£©2 | D”¢KNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗÓÄĻŹ”ÕņĘ½Ņ»øß2011£2012ѧğø߶žĻĀѧʌµŚŅ»“ĪŌĀæ¼»ÆѧŹŌĢā ĢāŠĶ£ŗ022
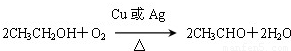
Ņ»¹ÉµµĒåĻćÄÜøųČĖŠÄæõÉńāłµÄøŠ¾õ£¬Ö÷ŅŖÓÉĻć¾«”¢¾Ę¾«ŗĶĖ®¹¹³ÉµÄĻćĖ®±øŹÜ°®ĆĄČĖŹæµÄĒąķł£®Ļć¾«ĄļĆęŗ¬ÓŠõ„ĄąĪļÖŹ£¬¹¤ŅµÉĻŅŌAĪŖÖ÷ŅŖŌĮĻĄ“ŗĻ³ÉŅŅĖįŅŅõ„£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾£®ĘäÖŠAŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ö£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ÓÖÖŖ£ŗ2CH3CHO£«O2![]() 2CH3COOH£®
2CH3COOH£®
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
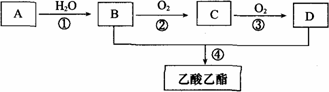
(1)Š“³öAµÄµē×ÓŹ½________£®
(2)B”¢D·Ö×ÓÄŚŗ¬ÓŠµÄ¹ŁÄÜĶÅ·Ö±šŹĒ________”¢________(ĢīĆū³Ę)£®
(3)Š“³öĻĀĮŠ·“Ó¦µÄ·“Ó¦ĄąŠĶ£ŗ¢Ł________£¬¢Ü________£®
(4)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł________£»
¢Ś________£»
¢Ü________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com