
| ���� | �۵㣨�棩 | �е㣨�棩 |
| �� | 841 | 1487 |
| �� | 920 | 3470 |

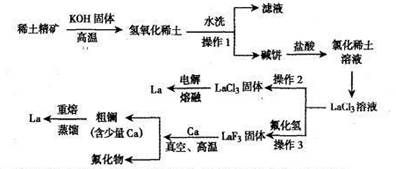
 3CaF2+2La����5��1487��3470��C����6��������
3CaF2+2La����5��1487��3470��C����6�������� 2La+ 3Cl2��.��β���к���������Ҫ�ü�Һ���գ��÷�Ӧ�����ӷ���ʽ��Cl2+2OH-=Cl-+ClO-+H2O����4����������ͼ��֪������ա����¹����еķ�Ӧ����ʽΪ3Ca+2LaF3
2La+ 3Cl2��.��β���к���������Ҫ�ü�Һ���գ��÷�Ӧ�����ӷ���ʽ��Cl2+2OH-=Cl-+ClO-+H2O����4����������ͼ��֪������ա����¹����еķ�Ӧ����ʽΪ3Ca+2LaF3 3CaF2+2La����5���ڴ����к�������Ca������Ca�ķе���1487�棬La�ķе���3470�棬���Ծ��ƹ������¶ȿ��Ʒ�Χ1487��3470�棻��6���ڸò�Ʒ��La������������{(69.709g-0.209g)��69.709g}��100%=99.7�����������ڷ�������
3CaF2+2La����5���ڴ����к�������Ca������Ca�ķе���1487�棬La�ķе���3470�棬���Ծ��ƹ������¶ȿ��Ʒ�Χ1487��3470�棻��6���ڸò�Ʒ��La������������{(69.709g-0.209g)��69.709g}��100%=99.7�����������ڷ�������

 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������7 | B��С��7 | C������7 | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��0.1 mol/L��NH4Cl��Һ�У�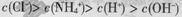 |
B��0.1mol/L��CH3COONa��Һ�У�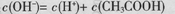 |
C��0.1 mol/LNa2S����Һ�У� |
D��pH=2��������pH=12�İ�ˮ�������Ϻ�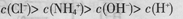 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c��NH4������ȵ�NH4HCO3��NH4HSO4��NH4Cl��Һ�У�c (NH4HSO4) ��c(NH4HCO3) ��c(NH4Cl) |
| B�����������Һ�м����������ᣬ�õ������Ի����Һ�У�C(Na+)��C(CH3COO-)��C(H+)��C(OH-) |
| C��1.0mol/LNa2CO3��Һ�У�C(OH-)=C(HCO3-)+C (H+)+2C(H2CO3) |
| D��ij��Ԫ�������ʽ��NaHA��Һ�У�C(H+)+C(Na+)=C(OH-)+C(HA-)+C(A2-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ����Һ�У�c(Cl��)��c(NH4��)��c(OH��)��c(H��) |
| B��̼������Һ�У�c(HCO3��)��c(CO32��)��c(H2CO3)��0.1 mol��L��1 |
| C����������Һ�У�c(OH��)��c(H��)��c(CH3COOH) |
| D��̼��������Һ�У�c(Na��)��c(OH��)��c(HCO3��)��c(CO32��)��c(H��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(K+)+c(H+) = c(HC2O4��)+c(OH��)+c(C2O42��) |
| B��c(HC2O4��)+c(C2O42��) =" 0.1" mol��L��1 |
| C��c(C2O42��) < c(H2C2O4) |
| D��c(K+) = c(H2C2O4)+c(HC2O4��)+c(C2O42��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
 2BO3(g)����H=��196.6kJ/mol
2BO3(g)����H=��196.6kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
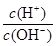 ��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ�ȵĹ�ϵ��ȷ����( )
��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ�ȵĹ�ϵ��ȷ����( )�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��Al Al2O3 Al2O3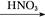 Al(NO3)3 Al(NO3)3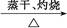 Al2O3 Al2O3 |
B��Cu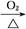 CuO CuO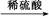 CuSO4��Һ CuSO4��Һ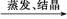 CuSO4��5H2O CuSO4��5H2O |
C��Fe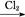 FeCl3 FeCl3 Fe(OH)3 Fe(OH)3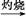 Fe2O3 Fe2O3 |
D��FeSO4��Һ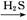 FeS FeS FeS FeS |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com