
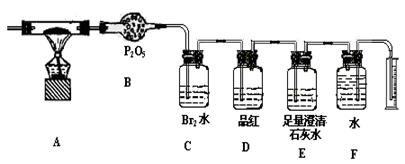
| A£®¹Ū²ģĖįŹ½µĪ¶Ø¹ÜŅŗĆꏱ£¬æŖŹ¼ø©ŹÓ£¬µĪ¶ØÖÕµćĘ½ŹÓ£¬Ėł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| B£®ÓƱź×¼ŃĪĖįµĪ¶ØĪ“ÖŖÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬ĖįŹ½µĪ¶Ø¹ÜĻ“¾»ŗó£¬Ć»ÓŠÓƱź×¼ŃĪĖįČóĻ“¶ųÖ±½Ó×°±ź×¼ŃĪĖįµĪ¶Ø¼īŅŗ£¬Ėł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| C£®ÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗÓĆ·ÓĢŖ×öÖøŹ¾¼ĮĖł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| D£®×öÖŠŗĶČČ²ā¶ØŹ±£¬ŌŚ“óŠ”Į½øöÉÕ±Ö®¼äƻӊµęĖéÅŻÄĖÜĮĻ(»ņÖ½Ģõ)Ėł²ā³öµÄÖŠŗĶČČŹżÖµ |


 ×Ö“Ź¾äĘŖÓėĶ¬²½×÷ĪÄ“ļ±źĻµĮŠ“š°ø
×Ö“Ź¾äĘŖÓėĶ¬²½×÷ĪÄ“ļ±źĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| A£®¶ØČŻŹ±ø©ŹÓŅŗĆę | B£®ÅäÖĘŹ±ČŻĮæĘæƻӊøÉŌļ |
| C£®×ŖŅĘŹ±Ć»ÓŠĻ“µÓÉÕ±ŗĶ²£Į§°ō | D£®³ĘĮæŹ±ķĄĀėĪ»ÖĆ·Å“ķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
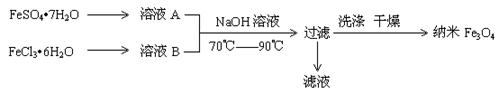
| A£®±£“ęČÜŅŗAŹ±£¬Ó¦¼ÓČėÉŁĮæĢś·Ū |
| B£®ĘäÖʱø·“Ó¦ĄąŠĶ²»ŹōÓŚŃõ»Æ»¹Ō·“Ó¦ |
| C£®Č”ĀĖŅŗ½ųŠŠŃęÉ«·“Ó¦£¬»šŃęĪŖ»ĘÉ« |
| D£®FeSO4”¤7H2O ŗĶ FeCl3”¤6H2OµÄĪļÖŹµÄĮæÖ®±Č×īŗĆÓ¦ĪŖ2”Ć1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½«5.85gNaCl¹ĢĢåČÜÓŚ1 LĖ®ÖŠæÉÅä³É0.1 mol”¤L-1µÄNaClČÜŅŗ |
| B£®¶ØČŻŹ±ŃöŹÓ¹Ū²ģŅŗĆę£¬ŹµŃé½į¹ūĘ«µĶ |
| C£®¹ĢĢåČܽāŗ󣬽«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæÖŠ£¬Č»ŗóĻņČŻĮæĘæÖŠÖ±½Ó¼ÓĖ®Ļ”ŹĶµ½æĢ¶ČĻß |
| D£®ÅäÖĘČÜŅŗŹ±ČŻĮæĘæÖŠŌĄ“ÓŠÉŁĮæÕōĮóĖ®²»»įÓ°ĻģŹµŃé½į¹ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
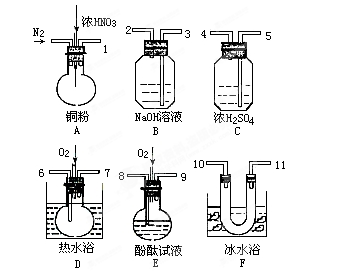
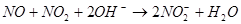
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®O2 | B£®H2 | C£®NO2 | D£®NO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H2 | B£®NH3 | C£®O2 | D£®NO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com