
U��V��W��X��Y��Z��ԭ������������������ֳ���Ԫ�ء�Y�ĵ�����W2��ȼ�յIJ����ʹƷ����Һ��ɫ��Z��WԪ���γɵĻ�����Z3W4���д��ԡ�U�ĵ�����W2��ȼ�տ�����UW��UW2�������塣X�ĵ�����һ�ֽ������ý�����UW2�о���ȼ�����ɺڡ������ֹ��塣��ش��������⣺
��V�ĵ��ʷ��ӵĽṹʽΪ_______________��XW�ĵ���ʽΪ____________��
ZԪ�������ڱ��е�λ������������������������������
��UԪ���γɵ�ͬ��������ľ������Ϳ����ǣ�����ţ�__________��
�� ԭ�Ӿ��� �� ���Ӿ��� �� ���Ӿ��� �� ��������
��U��V��W�γɵ�10�����⻯���У�U��V���⻯��е�ϵ͵��ǣ�д��ѧʽ��____________��V��W���⻯����ӽ��H+������ǿ���ǣ�д��ѧʽ��______________����һ�����ӷ���ʽ����֤����_______________________________��
��YW2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����VW���йط�Ӧ�����ӷ���ʽΪ��__________________________________________���ɴ˿�֪VW��YW2��ԭ�Խ�ǿ���ǣ�д��ѧʽ��______________________________��
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
| 1 |
| 202 |
| 3 |
| 202 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
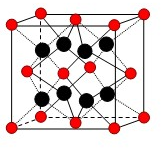 ��U��V��W��X��Y��Z��T����ǰ������Ԫ�أ����ǵ�ԭ���������������������Ϣ���±���
��U��V��W��X��Y��Z��T����ǰ������Ԫ�أ����ǵ�ԭ���������������������Ϣ���±���| Ԫ�ر�� | �� �� �� Ϣ |
| U | ��������������������Ӳ��� |
| V | ��̬ʱ�����ӷֲ��������ܼ��ϣ��Ҹ��ܼ��е���������� |
| W | �䵥���ǿ�������Ҫ�ɷ�֮һ���һ�ѧ�����൱�ȶ� |
| X | ��WԪ�ش���ͬһ���ڣ���X�ĵ�һ������С��W�ĵ�һ������ |
| Y | �䵥������ǿ�������� |
| Z | ZԪ�صĶ������������ԭ�ӵĵ��Ӳ�ṹ��ͬ |
| T | �ǵ�������Ԫ����δ�ɶԵ���������Ԫ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com