
��25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ�ζ�������ͼ��ʾ������˵����ȷ����

A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX
B�����ݵζ����ߣ��ɵ�Ka(HY)��10��6
C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��
c(X��)��c(Y��)��c(OH��)��c(Na+)��c(H��)
D��HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)��c(Y��)��c(Z��)��c(OH��)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��д��ȷ����
A����CaCl2��Һ��ͨ������CO2���壺 Ca2++CO2+H2O��CaCO3��+2H+
B��̼������Һ������������Һ��Ӧ�� HCO3��+H+��CO2��+H2O
C����Ba(OH)2��Һ�еμ�NH4HSO4��Һ���պó�����ȫ��
Ba2++2OH��+NH4++H++SO42����BaSO4��+NH3��H2O+ H2O
D������FeBr2��Һ��ͨ��������Cl2�� 2Fe2+��4Br-��3Cl2��2Fe3+��2Br2��6Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ����ѧ������ѧ��һ�����л�ѧ�Ծ��������棩 ���ͣ������
ij��ɫ����Һ�п��ܴ����������������еļ��֣�Na����Mg2����Ca2����Fe2����Cu2����OH����Cl����CO32-��HCO3-��NO��SO42-������д���пհף�
��1�������κ�ʵ��Ϳ��Կ϶���Һ�в����ڵ������� ��
��2��ȡ����ԭ��Һ�μӼ��η�̪��Һ����Һ���ɫ����ʵ�������˵��ԭ��Һ�п϶������ڵ������� ��
��3����ȡ����ԭ��Һ��εμ������������������塢�������ɣ��ټ���BaCl2��Һ���а�ɫ�������ɡ���ʵ�������˵��ԭ��Һ�п϶������ڵ����ӻ��� ��
��4������3��ʵ�����û��Һ���ã�ȡ�����ϲ���Һ������AgNO3��Һ���а�ɫ�������ɡ�
��������ʵ���ƶϣ�ԭ��Һ�п϶����ڵ�������______________�����ܿ϶����ڵ�������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ϳ����и߶�12���¿���ѧ���������棩 ���ͣ�ѡ����
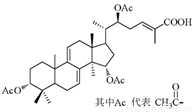
�� ��ȫת��Ϊ
��ȫת��Ϊ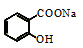 �ķ����ǣ� ��
�ķ����ǣ� ��
A����������NaOH��Һ���Ⱥ���ͨ��CO2
B������Һ���ȣ�ͨ��������HCl COONa OH
C����ϡ���Ṳ�Ⱥ���������Na2CO3
D����ϡ���Ṳ�Ⱥ���������NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��������������ʵ����ѧ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
��֪��2FeCl3��2KI===2FeCl2��2KCl��I2��2FeCl2��Cl2===2FeCl3��2KMnO4��16HCl===2KCl��2MnCl2��5Cl2����8H2O����ij��Һ����Fe2���� I����Cl����Ҫ������ȥI������Ӱ��Fe2����Cl�����ɼ�����Լ���( )
A��Cl2 B��KMnO4 C��FeCl3 D��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�������һ��ѧ����һ���չٿ���һ���ۻ�ѧ���������棩 ���ͣ�ѡ����
���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ������ʾ��
Ԫ�ش��� | X | Y | Z | W |
ԭ�Ӱ뾶/pm | 160 | 143 | 70 | 66 |
��Ҫ���ϼ� | +2 | +3 | +5.+3.-3 | -2 |
����������ȷ���ǣ� ��
A��X��YԪ�صĽ����ԣ�X<Y
B��һ�������£�Z������W�ij�������ֱ������ZW2
C��Y������������Ӧ��ˮ����������ϡ��ˮ
D��һ�������£�W���ʿ��Խ�Z���ʴ����⻯�����û�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���㽭��Ҧ��ѧ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��У�о���������������ͼ���������֥�ᵥ��ķ��ӽṹͼ����ͼ��ʾ�����й��ڸ��л����˵������ȷ����
A������Br2�����ӳɷ�Ӧ
B���ܷ���������Ӧ
C���ܷ���ˮ�ⷴӦ
D����������7 ������̼ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ����ׯ���и߶�10��ѧ����黯ѧ���������棩 ���ͣ�ѡ����
��ͼ��ʾ��װ���ж�ʢ��0.1mol��L-1 ��NaCl ��Һ������һ��ʱ���װ���е��Ŀ���ͬ��пƬ��ʴ�����ɿ쵽������ȷ˳���ǣ��� ��
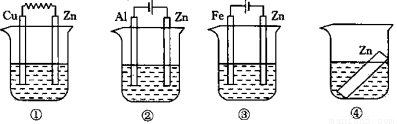
A���٢ڢۢ� B���٢ڢܢ� C���ڢ٢ܢ� D���ۢ٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ�߶������в��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ������Ҫ����0.50 mol/L NaCl��Һ480 mL����ʹ��NaCl�������ƣ������в������������ʵ������֣���ʹ��������������
��.��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������__________��_________________�Լ�����������Ƭ��ֽ��
��2�����㡣���Ƹ���Һ��ȡNaCl����________ g��
��3��������
����ƽ��ƽ�� �ڳ����� �۳�����ϣ���ҩƷ�����ձ��С�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����___________________��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ��________��������Ҫϴ���ձ�2��3����Ϊ��_______________________��
��6�����ݡ�ҡ�ȡ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�У������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
��8�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�______(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
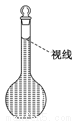
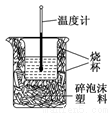
��ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
��1����ͼ��������δ������������________________��________________��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ���_________________��
��3������0.5 mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��________(�ƫ����ƫС���������䡱)��ԭ����_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com