
 CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3�� �����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl
�����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�� | B�� | C�� | D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��7.8gNa2O2������������������֮��һ��Ϊ0.3NA |
| B�������£�14.0g��ϩ�Ͷ�ϩ�Ļ�������к��е���ԭ����ĿΪNA |
| C��0.1mol Fe��0.1mol Cl2�г��ȼ�գ�ת�Ƶĵ�����Ϊ0.3NA |
| D�����³�ѹ�£�1mol�������ĵ�������Ϊ7 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʵ�����ȵ�H2O��D2O���е������� |
| B��20 mL NH3��30 mL O2������ԭ���� |
| C�������ʵ�����Na2O��Na2O2�к��е����������� |
| D�������ʵ������ƺ�ͭ�ֱ���������ȫ��Ӧ��ת�Ƶĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��80��ʱ��1 L pH��1��������Һ�У�����0.2NA��H�� |
| B��4.6 g Na��ȫת����Na2O��Na2O2�Ļ���������������������Ϊ0.1NA |
| C����״���£�2.24 L Cl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA |
| D��300 mL 2 mol��L��1������Һ������������Ϊ0.6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

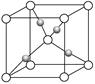


| A������ӳ�������105 ��Ĺ�����ֻ��������� |
| B�������������γ���λ����4��ˮ����ͬʱʧȥ |
| C��120 ��ʱ��ʣ�����Ļ�ѧʽ��CuSO4��H2O |
| D������������ʧˮʱ���˷�����������С��ͬ�������е�ˮ���ӿ��Է�Ϊ3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.4 mol��L��1 | B��0.3 mol��L��1 |
| C��0.2 mol��L��1 | D��0.1 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1 mol Na2O2���������4NA������ |
| B��0.1 mol AlCl3��ȫˮ��ת��Ϊ������������,����0.1NA������ |
| C�����³�ѹ��16 g O2��O3������庬��NA����ԭ�� |
| D��1 mol/L��CaCl2��Һ�к�Cl-����ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com