
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
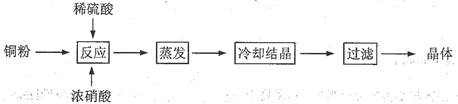
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 ��ش��������⣺
��ش��������⣺ ��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
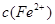 �ı仯��ͼ��ʾ���������ӷ���ʽ�������( )
�ı仯��ͼ��ʾ���������ӷ���ʽ�������( )
| A��0��1 : Fe+ NO3��+4H+=Fe3++ NO��+2H2O | B��1��2 : Fe+2Fe3+ =3Fe2+ |
| C��2��3 : Fe+Cu2+��Fe2++Cu | D��0��3 : 3Fe+2Fe3++2Cu2+ =5Fe2++2Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��Ũ���� | B��ϡ���� | C������������Һ | D������ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 05 mol/L��NaOH��Һ������һ����Һ�еμ�0.600 mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������
05 mol/L��NaOH��Һ������һ����Һ�еμ�0.600 mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ����������������� Һ������仯����ͼ��ʾ��
Һ������仯����ͼ��ʾ��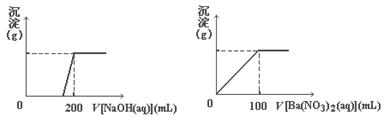
 ����ַ�Ӧ���ڱ�״��������NO�������ʣ��������������±���������Ļ�ԭ����ֻ��NO����
����ַ�Ӧ���ڱ�״��������NO�������ʣ��������������±���������Ļ�ԭ����ֻ��NO����| ʵ���� | �� | �� | �� | �� |
| ϡ�������/mL | 100 | 200 | 300 | 400 |
| ʣ���������/g | 9.0 | 4.8 | 0 | 0 |
| NO���/L | 1.12 | 2.24 | 3.36 | V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.15mol/L | B��0.3mol/L | C��0.225mol/L | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO3�� | B��Fe3�� | C��SO42�� | D��Mg2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com