
��13�֣���д���пհף�
��1��34g NH3����__________molԭ�ӣ�0.1molH2S��Լ��____ _����ԭ�ӡ�ͬ��ͬѹ�£��������NH3��H2S�����������Ϊ____________�����еķ�����Ŀ֮��Ϊ ����������NH3��H2S�з�����Ŀ֮��Ϊ_________��
��2���ڱ�״���£�35.5g Cl2�����Լ��_________L��������������ȫ���������Ȼ��������������ʵ�����_________mol�������ɵ��Ȼ�����������1000 gˮ�У��õ��ܶ�Ϊa g��cm-3�����ᣬ�����������ʵ���Ũ����_____________mol/L��
��3��������500mL 0.2mol/L Na2CO3��Һ����Ҫ����ƽ����Na2CO3��10H2O��������Ϊ ��������õ�������Һ��ȡ��50mL��һ�Լ�ƿ�У���Ҫ�������ϱ�ǩ����ǩ�ϵ������� �����ٴ���ȡ��10mL��Һ��ˮϡ����20mL�������Һ��Na+�����ʵ���Ũ��Ϊ ��
��13�֣���1��8 ��1.204��1023 �� 1��2 ��1��1 ��2��1 ��
��2��11.2 �� 0.5 ��0.965 ��
��3��28.6 g ��0.2mol/L Na2CO3��Һ �����ޡ���Һ����1�֣��� 0.2mol/L�����»��ߴ�ÿ��2�֣�����ÿ��1�֡�����д��λ����©д��λ���֡���
�����������������ʵ��������ʵ���Ũ�ȡ���Һ���ƵĻ�������Ͳ���
(2) 1 mol���Ȼ����ܽ���1000gˮ�У�������������Ϊ�� =1.0365L�������������ʵ���Ũ����
=1.0365L�������������ʵ���Ũ����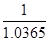 =0.965 mol/L
=0.965 mol/L



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 +Br2
+Br2| Fe |
 +HBr
+HBr +Br2
+Br2| Fe |
 +HBr
+HBr�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
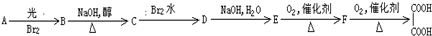
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com