
��9�֣����������Ҫ�ɷ��� ����������
���������� ��
�� ��
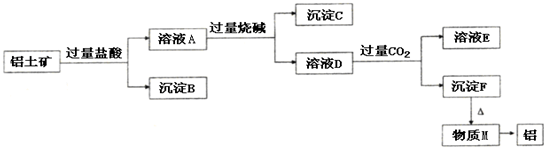
�� ����ҵ�ϴ�����������ȡ���ɲ������¹������̣�
����ҵ�ϴ�����������ȡ���ɲ������¹������̣�

��ش��������⣺
��1��ͼ���漰������Һ�������ʵ�鷽����____________����������ƣ���
��2������B�ijɷ���____________���ѧʽ����ͬ����B��������������ͨ��������ѧ����ʽ��____________________��____________________������C�ijɷ���____________����ҺD�д��ڽ϶����������____________��
��3������Fת��Ϊ����M�Ļ�ѧ����ʽΪ________________________��������M��ȡ���Ļ�ѧ����ʽΪ________________________����ҺD��ͨ����� ���ɳ���F�����ӷ���ʽΪ________________________��
���ɳ���F�����ӷ���ʽΪ________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ����ʯ |
| ||
| ����ʯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ʊ����Ĺ������̣�
����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ʊ����Ĺ������̣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com