
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
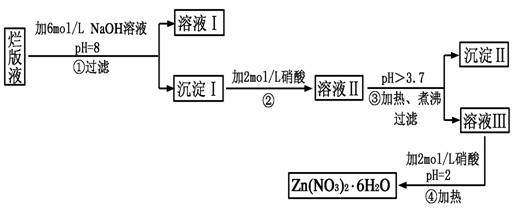
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
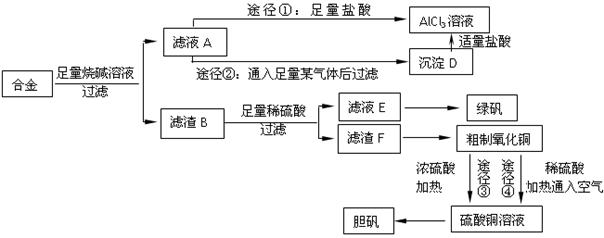
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
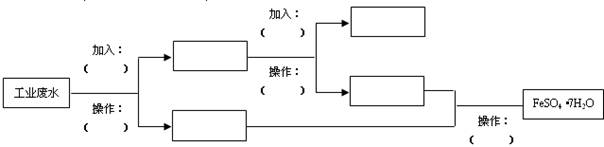
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
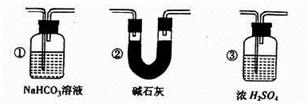
��
�鿴�𰸺ͽ���>>
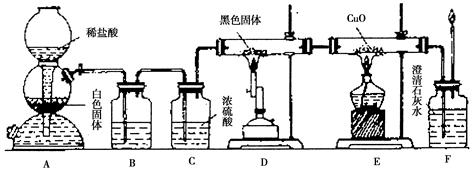
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | NaOH��ʼ���� | NaOH�յ���� |
| ��һ�� | 0.40mL | 18.50mL |
| �ڶ��� | 1.30mL | 18.05mL |
| ������ | 3.10mL | 21.20mL |
 ɫ�� ɫ��
ɫ�� ɫ��| A���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ |
| B��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ |
| C���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ |
| D��δ�ñ�Һ��ϴ��ʽ�ζ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�����CoCl2�ĸ����ɫ�轺����ɫ���ڳ�ʪ�Ŀ����б�ۺ�ɫ�����ڸ���Ŀ������ָֻ�Ϊ��ɫ |
| B����ɫ��Ӧʵ���У���˿��պȡ������Һǰ��Ӧ����ϡ����ϴ�������� |
| C���к͵ζ�ʵ���У�������ʢ�Ŵ�����Һ����ƿϴ����δ�����Ӱ��ⶨ��� |
| D��ֽ�����У���ֽ��Ϊ�̶��࣬չ������Ϊ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��B�е������в������� | B��B�е������� �γ�һ��ˮ�� �γ�һ��ˮ�� |
| C������Ƭ���ڱ�� | D��п����ʴ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com