
ŅŃÖŖijČÜŅŗÖŠÖ»“ęŌŚOH-”¢H+”¢NH4+”¢Cl-ĖÄÖÖĄė×Ó£¬Ä³Ķ¬Ń§ĶĘ²āĘäĄė×ÓÅØ¶Č“óŠ”Ė³ŠņÓŠŅŌĻĀ¼øÖÖ
¢Łc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©
¢Śc£ØCl-£©£¾c£ØNH4+£©£¾c£ØOH-£©£¾c£ØH+£©
¢Ūc£ØNH4+£©£¾c£ØCl-£©£¾c£ØOH-£©£¾c£ØH+£©
¢Üc£ØCl-£©£¾c£ØH+£©£¾c£ØNH4+£©£¾c£ØOH-£©
£Ø1£©ÉĻŹö¹ŲĻµŅ»¶Ø²»ÕżČ·µÄŹĒ_______________£ØĢīŠņŗÅ£©£»
£Ø2£©ČōČÜŅŗÖŠÖ»ÓŠŅ»ÖÖČÜÖŹ£¬ŌņøĆČÜÖŹĪŖ__________£¬øĆČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”¹ŲĻµĪŖ_______£ØĢīŠņŗÅ£©£»
£Ø3£©Čō¹ŲĻµ¢ŪÕżČ·£¬ŌņČÜŅŗÖŠČÜÖŹĪŖ_________£»
£Ø4£©ČōĖÄÖÖĄė×ÓÅØ¶Č¹ŲĻµÓŠc£ØNH4+£©=c£ØCl-£©£¬ŌņøĆČÜŅŗĻŌ_________£ØĢī”°ĖįŠŌ”±”¢”°¼īŠŌ”±”¢”°ÖŠŠŌ”±£©”£
£Ø5£©25”ę£¬pH=aµÄŃĪĖįVamLÓėpH=14-aµÄ°±Ė®VbmL»ģŗĻ£¬ČōČÜŅŗĻŌÖŠŠŌ£¬ŌņVa__________Vb£ØĢī£¾”¢£¼”¢=”¢ĪŽ·ØČ·¶Ø£©”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016½ģɽ¶«Ź”øßČżÉĻѧʌµŚ¶ž“ĪÕļ¶Ļ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijĶ¬Ń§ÓĆĻĀĮŠ×°ÖĆÖʱø²¢¼ģŃéCl2µÄŠŌÖŹ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
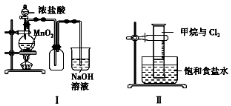

A£®¢ńĶ¼ÖŠ£ŗČē¹ūMnO2¹żĮ棬ÅØŃĪĖį¾Ķæɱ»Č«²æĻūŗÄ
B£®¢ņĶ¼ÖŠ£ŗĮæĶ²ÖŠ·¢ÉśĮĖČ”“ś·“Ó¦
C£®¢óĶ¼ÖŠ£ŗÉś³ÉĄ¶É«µÄŃĢ
D£®¢ōĶ¼ÖŠ£ŗøÉŌļµÄÓŠÉ«²¼ĢõĶŹÉ«£¬ĖµĆ÷ĀČĘųÓŠĘư׊Ō
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğ½ĖÕŹ”ø߶žÉĻĘŚÖŠ±ŲŠŽ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ÓŠAl”¢CuO”¢Fe2O3×é³ÉµÄ»ģŗĻĪļ¹²10.0g£¬·ÅČė500mLijÅØ¶ČµÄŃĪĖįČÜŅŗÖŠ£¬»ģŗĻĪļĶźČ«Čܽā£¬µ±ŌŁ¼ÓČė250mL 2.00mol/LµÄNaOHČÜŅŗŹ±£¬µĆµ½³Įµķ×ī¶ą”£ÉĻŹöŃĪĖįČÜŅŗµÄÅضČĪŖ£Ø £©
A£®0.500mol/L B£®1.00mol/L C£®2.00mol/L D£®3.00mol/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğ½ĖÕŹ”øßŅ»ÉĻĘŚÖŠ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ĻĀĶ¼ŹĒŹµŃéŹŅÖʱøĀČĘų²¢½ųŠŠŅ»ĻµĮŠĻą¹ŲŹµŃéµÄ×°ÖĆ£Ø¼Š³Ö¼°¼ÓČČŅĒĘ÷ŅŃĀŌ£©”£
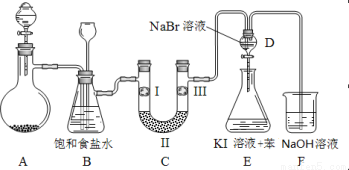
£Ø1£©ÖʱøĀČĘųŃ”ÓƵÄŅ©Ę·ĪŖ¹ĢĢ嶞Ńõ»ÆĆĢŗĶÅØŃĪĖį£¬ŌņĻą¹ŲµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ£ŗ ”£×°ÖĆBÖŠ±„ŗĶŹ³ŃĪĖ®µÄ×÷ÓĆŹĒ_________£»Ķ¬Ź±×°ÖĆBŅąŹĒ°²Č«Ę棬¼ą²āŹµŃé½ųŠŠŹ±CÖŠŹĒ·ń·¢Éś¶ĀČū£¬ĒėŠ“³ö·¢Éś¶ĀČūŹ±BÖŠµÄĻÖĻó_________”£
£Ø2£©×°ÖĆCµÄŹµŃéÄæµÄŹĒŃéÖ¤ĀČĘųŹĒ·ń¾ßÓŠĘư׊Ō£¬ĪŖ“ĖCÖŠI”¢II”¢IIIŅĄ“Ī·ÅČė_______”£
a | b | c | d | |
I | øÉŌļµÄÓŠÉ«²¼Ģõ | øÉŌļµÄÓŠÉ«²¼Ģõ | ŹŖČóµÄÓŠÉ«²¼Ģõ | ŹŖČóµÄÓŠÉ«²¼Ģõ |
II | ¼īŹÆ»Ņ | ¹č½ŗ | ÅØĮņĖį | ĪŽĖ®ĀČ»ÆøĘ |
III | ŹŖČóµÄÓŠÉ«²¼Ģõ | ŹŖČóµÄÓŠÉ«²¼Ģõ | øÉŌļµÄÓŠÉ«²¼Ģõ | øÉŌļµÄÓŠÉ«²¼Ģõ |
£Ø3£©Éč¼Ę×°ÖĆD”¢EµÄÄæµÄŹĒ±Č½ĻĀČ”¢ä唢µāµ„ÖŹµÄŃõ»ÆŠŌĒæČõ”£µ±ĻņDÖŠ»ŗ»ŗĶØČėÉŁĮæĀČĘųŹ±£¬æÉŅŌ擵½ĪŽÉ«ČÜŅŗÖš½„±äĪŖ__________É«£¬ĖµĆ÷_________________________”£
“ņæŖ»īČū£¬½«×°ÖĆDÖŠÉŁĮæČÜŅŗ¼ÓČė×°ÖĆEÖŠ£¬Õńµ“”£¹Ū²ģµ½µÄĻÖĻóŹĒ___________”£
£Ø4£©×°ÖĆFÖŠÓĆ×ćĮæµÄNaOHČÜŅŗĪüŹÕÓąĀČ£¬ŹŌŠ“³öĻąÓ¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğĮÉÄžŹ”ø߶žÉĻĘŚÖŠ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ņ»°ü»ģÓŠŌÓÖŹµÄĢ¼ĖįÄĘ¹ĢĢ壬ĘäŌÓÖŹæÉÄÜŹĒBa(NO3)2”¢KCl¼°NaHCO3ÖŠµÄŅ»ÖÖ»ņĮ½ÖÖ£¬½ńȔѳʷČÜÓŚŹŹĮæĖ®µĆµ½³ĪĒåČÜŅŗ”£ĮķČ”5.3gѳʷ£¬¼ÓČė×ćĮæµÄŃĪĖį£¬ŹÕ¼Æµ½2.2gCO2£¬ŌņĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ
A£®ŃłĘ·ÖŠÖ»ŗ¬ÓŠŅ»ÖÖŌÓÖŹNaHCO3
B£®ŃłĘ·ÖŠŗ¬ÓŠÓŠKCl£¬²»ŗ¬ÓŠNaHCO3
C£®ŃłĘ·ÖŠŗ¬ÓŠBa(NO3)2ŗĶNaHCO3
D£®ŃłĘ·ÖŠŗ¬ÓŠKClŗĶNaHCO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğĞĻÄÖŠĪĄŅ»ÖŠø߶žÉĻµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠČÜŅŗÖŠH+ÅضČĪŖ0.1 mol•L-1µÄŹĒ
A£®0.1 mol•L-1µÄCH3COOH B£®0.1 mol•L-1µÄNaHSO4
C£®0.1 mol•L-1µÄNaHCO3 D£®0.05 mol•L-1µÄH2SO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğĞĻÄÖŠĪĄŅ»ÖŠøßŅ»ÉĻµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŅŃÖŖŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢ÉśĻĀĮŠ·“Ó¦£ŗN2+3Mg=Mg3N2£Ø¹Ģ£©£¬æÕĘų»ŗ»ŗĶعżĻĀĶ¼×°ÖĆŹ±£¬ŅĄ“Ī³żČ„µÄĘųĢåŹĒ£ØÅØĮņĖį¾ßÓŠĪüĖ®ŠŌ£©
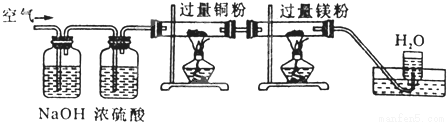
A£®CO2”¢N2”¢H2O”¢O2 B£®H2O”¢CO2”¢N2”¢O2
C£®CO2”¢H2O”¢O2”¢N2 D£®N2”¢O2”¢CO2”¢H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016ѧğÖŲĒģŹŠø߶žÉĻĘŚÖŠ²āŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ»ÆѧÓĆÓļÕżČ·µÄŹĒ( )
A£® NaHCO3 µÄµēĄė£ŗ
B£®HS- µÄµēĄė£ŗHS”Ŗ+H2O H3O++S2-
H3O++S2-
C£®H3PO4µÄµēĄė£ŗ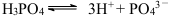
D£®ČŪȌדĢ¬ĻĀNaHSO4µÄµēĄė£ŗ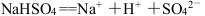
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015-2016Ń§ÄźÉ½¶«Ź”øßŅ»ÉĻ12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠĪļÖŹ¼ä·“Ó¦µÄĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ£Ø £©
A£®ĻąĶ¬ĪļÖŹµÄĮæÅØ¶ČµÄFeI2ČÜŅŗÓėäåĖ®µČĢå»ż»ģŗĻ 2Fe2++2I-+2Br2=2Fe3++I2+4Br-
B£®Ba(OH)2ČÜŅŗÖŠ¼ÓČė¹żĮæµÄNaHSO4ČÜŅŗ Ba2++OH-+H++SO42-=H2O+BaSO4”ż
C£®ĒāŃõ»ÆŃĒĢśČÜŅŗÓŚĻ”ĻõĖįÖŠ Fe(OH)2+2H+=Fe2++2H2O
D£®ĻņĘÆ°×·ŪČÜŅŗÖŠĶØČėÉŁĮæµÄ¶žŃõ»ÆĢ¼ Ca2++2ClO-+CO2+H2O=CaCO3”ż+2HClO
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com