
ijУ��ѧ��ȤС����ʵ�����з���һƿ��Һ����ǩ�ϱ��С�CaCl2,0.1 mol��L��1���������������Ǹ�С���Ա�Ը���Һ����������ȷ����(����)
A������1 L����Һ���ɽ�0.1 mol CaCl2����1 Lˮ��
B��Ca2����Cl�������ʵ���Ũ�ȶ���0.1 mol��L��1
C�����Լ�ƿ��ȡ����Һ��һ�룬��������Һ�����ʵ���Ũ��Ϊ0.05 mol��L��1
D������ƿ��Һϡ��һ������������Һ��c(Cl��)Ϊ0.1 mol��L��1
��������A�������Һ���������1 L������B�Cl����Ũ����0.2 mol��L��1��Ca2����Ũ����0.1 mol��L��1������C���Һ���о�һ�ԣ�������Һ��Ũ����Ϊ0.1 mol��L��1������D���Һϡ��һ���������Ϊԭ����2�������ʵ����ʵ������䣬��ԭ��Һ�����ΪV L��ϡ��ǰ��Cl�����ʵ���Ϊ2��0.1 mol��L��1��V L��0.2V mol��ϡ�ͺ�Cl�����ʵ���Ũ��Ϊ0.2V mol/2V L��0.1 mol��L��1����ȷ��
�𰸣���D



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڼס��ҡ���������������װ�õ��й��÷������в���������
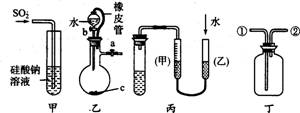
A����װ�ã�������֤����ķǽ����Աȹ�ǿ
B����װ�ã���Ƥ�ܵ���������ʹˮ˳������
C����װ�ã���ͼʾ�ķ����ܼ���װ�õ�������
D����װ�ã�����ƿ����װ��ij��Һ���ռ�NO����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(NiMH)Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������͡�NiMH�е�M��ʾ���������Ͻ𡣸õ���ڳ������е��ܷ�Ӧ����ʽ�ǣ�Ni(OH)2��M = NiOOH��MH��֪��6NiOOH��NH3��H2O��OH��=6Ni(OH)2��NO2��������˵����ȷ����
A. NiMH ��طŵ�����У������ĵ缫��ӦʽΪ��NiOOH��H2O��e��= Ni(OH)2��OH��
B. ��������OH�����Ӵ�����������Ǩ��
C. �������������ĵ缫��Ӧʽ��H2O��M��e��= MH��OH����H2O�е�H��M��ԭ
D. NiMH����п�����KOH��Һ����ˮ����Ϊ�������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���£�a g����A��b g����B�ķ�������ͬ������˵���У�����ȷ����(����)
A��A��B����Է�������֮��Ϊa��b
B��ͬ��ͬѹ�£�A��B���ܶ�֮��Ϊb��a
C��ͬ������A��B�����ʵ���֮��Ϊb��a
D��ͬ��ͬѹ�£�ͬ�����A��B������֮��Ϊa��b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ũ��Ϊ2 mol��L��1��NaOH��Һ����ȷ������(����)
A����2 Lˮ������80 g NaOH B��80 g NaOH����ˮ��ɵ���Һ
C��1 L��Һ������80 g NaOH D��2 L��Һ������80 g NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ�������ȷ����(����)
A��������ڼ�
B��CuSO4����������
C�����ܵ����H���Ļ������������
D����������һ�����н���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�����ʣ���ˮ���ڿ������������ܶ�����̼
�����ᡡ����ʯ��[Ca(OH)2]���ߵ���(CuSO4��5H2O)����NaOH��Һ�����ʽ̼��ͭ[Cu2(OH)2CO3]������������(NaHSO4)
(1)���ڻ�������________��
(2)�������������________��
(3)���ڼ����______��
(4)���������______��
(5)�����ε���________��(���Ͽո������ʵ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
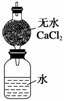
ijУѧ������С��Ϊ�ⶨNaԪ�ص����ԭ����������Ƶ�װ����ͼ����װ��(����ˮ�����)��������Ϊa g����ʵ����ȡ��b g(������)���Ʒ���ˮ�У�����ƿ������ȫ��Ӧ���ٳ�����װ�õ�������Ϊc g���Իش�
(1)ʵ����ȡ��һС���ƣ���Ҫ�õ�_________________________________________��
(2)�˸���ܵ�������___________________________________________________��
�����ƿ��м����������������������ԭ���������ʵ�����ԭ������__________(�ƫ����ƫС�����䡱����ͬ)��
(3)�м�ͬѧ������ͼ�и����������һͬ������ܣ���Ŀ����_____________�������Ƶ����ԭ�������ı���ʽΪ__________��
(4)����ͬѧΪ���������ж�����Ѹ�ٲ���ƫ��ֽ����ڼ���ƿ���ټ���һ����ú�ͣ���Ŀ����___________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��Һ��ֻ��������8�������е�ij���֣�Mg2����H����Ag����Na����Cl����HCO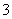 ��OH����NO
��OH����NO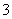 ����֪����Һ��������Ӧ�ų��������Իش��������⣺
����֪����Һ��������Ӧ�ų��������Իش��������⣺
(1)����Ӧ������Al3��������Һ�п��ܴ��ڵ�������__________________��һ�����ڵ���
����________________��һ�������ڵ�������____________����Ӧ�����ӷ���ʽΪ
________________________________________________________________________
________________________________________________________________________��
(2)����Ӧ������[Al(OH)4]��������Һ�п��ܴ��ڵ�������____________��һ�����ڵ�������____________��һ�������ڵ�������____________����Ӧ�����ӷ���ʽΪ__
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com