
…ηNA¥ζ±μΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)
AΘ°1 molΦΉΜυ(ΓΣCH3)÷–Κ§”–ΒγΉ” ΐΈΣ7NA
BΘ°25 Γφ ±Θ§pHΘΫ7ΒΡNH4ClΚΆNH3ΓΛH2OΒΡΜλΚœ»ή“Κ÷–Θ§OHΘ≠ ΐΈΣ10Θ≠7NA
CΘ°4.6 g““¥Φ÷–Κ§”–Ι≤ΦέΦϋ ΐΡΩΈΣ0.8NA
DΘ°ΝΫΖίΨυΈΣ2.7 gΒΡ¬Ν―υΤΖΖ÷±π”κ100 mL≈®Ε»ΨυΈΣ2 molΓΛLΘ≠1ΒΡ―ΈΥαΚΆ«β―θΜ·ΡΤ»ή“Κ≥δΖ÷Ζ¥”ΠΘ§ΉΣ“ΤΒγΉ” ΐΨυΈΣ0.3NA

| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ6ΝΖΥ°»ή“Κ÷–ΒΡάκΉ”ΤΫΚβΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
(1)pHΘΫ13ΒΡCH3COONa»ή“ΚΦ”Υ°œΓ Ά100±ΕΚσΘ§pH________11(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)‘≠“ρ «______________________________________________(”ΟάκΉ”ΖΫ≥Χ ΫΚΆ±Ί“ΣΒΡΈΡΉ÷ΥΒΟς)ΘΜpHœύΒ»ΒΡNaOH»ή“Κ”κCH3COONa»ή“ΚΘ§Ζ÷±πΦ”»»ΒΫœύΆ§ΒΡΈ¬Ε»ΚσCH3COONa»ή“ΚΒΡpH________NaOH»ή“ΚΒΡpH(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)ΘΜ
(2)pHœύΒ» ±Θ§ΔΌNH4ClΓΓΔΎ(NH4)2SO4ΓΓΔέNH4HSO4»ΐ÷÷»ή“Κ÷–c(NH4+)”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________ΘΜ
(3)Β»ΧεΜΐΓΔΒ»≈®Ε»ΒΡ«β―θΜ·ΡΤ”κ¥ΉΥαΜλΚœΚσ»ή“Κ≥ ________–‘Θ§»ή“Κ÷–c(NaΘΪ)________c(CH3COOΘ≠)(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)ΘΜpHΘΫ13ΒΡ«β―θΜ·ΡΤ”κpHΘΫ1ΒΡ¥ΉΥαΒ»ΧεΜΐΜλΚœΚσ»ή“Κ≥ ________–‘Θ§»ή“Κ÷–c(NaΘΪ)________c(CH3COOΘ≠)(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)ΘΜ
(4)ΫΪΈο÷ ΒΡΝΩ≈®Ε»œύΆ§ΒΡ―ΈΥα”κΑ±Υ°ΜλΚœΚσΘ§»ή“Κ÷–ΒΡc(NH4+)ΘΫc(ClΘ≠)Θ§‘ρΜλΚœΚσ»ή“Κ≥ ________–‘Θ§―ΈΥαΒΡΧεΜΐ________Α±Υ°ΒΡΧεΜΐ(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)ΘΜ
(5)NaHSO4‘ΎΥ°÷–ΒΡΒγάκΖΫ≥Χ ΫΈΣNaHSO4=NaΘΪΘΪHΘΪΘΪSO42ΓΣΓΘΗΟ»ή“Κ÷–c(HΘΪ)_________________________c(OHΘ≠)ΘΪc(SO42ΓΣ)(ΧνΓΑ>Γ±ΓΑΘΫΓ±ΜρΓΑ<Γ±)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ3ΝΖΜ·―ßΖ¥”Π”κΡήΝΩ±δΜ·ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“―÷ΣΘΚΔΌH2O(g)=H2O(l) ΠΛH1ΘΫΘ≠Q1 kJΓΛmolΘ≠1
ΔΎC2H5OH(g)=C2H5OH(l) ΠΛH2ΘΫΘ≠Q2 kJΓΛmolΘ≠1
ΔέC2H5OH(g)ΘΪ3O2(g)=2CO2(g)ΘΪ3H2O(g) ΠΛH3ΘΫΘ≠Q3 kJΓΛmolΘ≠1
»τ Ι23 g“ΚΧ§ΈόΥ°ΨΤΨΪΆξ»Ϊ»Φ…’Θ§ΉνΚσΜ÷Η¥ΒΫ “Έ¬Θ§‘ρΖ≈≥ωΒΡ»»ΝΩΈΣ(ΒΞΈΜΘΚkJ)(ΓΓΓΓ)
AΘ°Q1ΘΪQ2ΘΪQ3
BΘ°1.5Q1Θ≠0.5Q2ΘΪ0.5Q3
CΘ°0.5Q1Θ≠1.5Q2ΘΪ0.5Q3
DΘ°0.5(Q1ΘΪQ2ΘΪQ3)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ2ΝΖ―θΜ·ΜΙ‘≠Ζ¥”ΠΚΆάκΉ”Ζ¥”ΠΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
Ϋπ τν―(Ti)–‘Ρή”≈‘ΫΘ§±Μ≥ΤΈΣΦΧΧζΓΔ¬Ν÷°ΚσΒΡΓΑΒΎ»ΐΫπ τΓ±ΓΘΙΛ“Β…œ“‘ΫπΚλ ·ΈΣ‘≠Νœ÷Τ»ΓTiΒΡΖ¥”ΠΈΣ
(1)aTiO2ΘΪbCl2ΘΪcC aTiCl4ΘΪcCO
aTiCl4ΘΪcCO
(2)TiCl4ΘΪ2Mg TiΘΪ2MgCl2
TiΘΪ2MgCl2
ΙΊ”ΎΖ¥”Π(1)ΓΔ(2)ΒΡΖ÷Έω≤Μ’ΐ»ΖΒΡ «
ΔΌTiCl4‘ΎΖ¥”Π(1)÷– «ΜΙ‘≠≤ζΈοΘ§‘ΎΖ¥”Π(2)÷– «―θΜ·ΦΝ
ΔΎCΓΔMg‘ΎΖ¥”Π÷–ΨυΈΣΜΙ‘≠ΦΝΘ§ΖΔ…ζΜΙ‘≠Ζ¥”ΠΓΓΔέΟΩ…ζ≥…19.2 g TiΘ§Ζ¥”Π(1)ΓΔ(2)÷–Ι≤ΉΣ“Τ4.8 mol eΘ≠ΓΓΔήaΘΫ1Θ§bΘΫcΘΫ2
AΘ°ΔΌΔΎΔήΓΓΓΓΓΓΓΓBΘ°ΔΎΔέΔή CΘ°ΔέΔή DΘ°ΔΎΔέ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ1ΝΖΈο÷ Ζ÷άύΜ·―ß”Ο”οΚΆ≥Θ”ΟΦΤΝΩΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Ε‘―άΗύΒΡΧΫΨΩ“Σ”ΟΒΫ–μΕύΜ·―ß÷Σ ΕΓΘ
(1)œ¬±μΝ–≥ωΝΥ»ΐ÷÷―άΗύΒΡΡΠ≤ΝΦΝΘ§«κ‘Ύ±μ÷–Χν–¥»ΐ÷÷ΡΠ≤ΝΦΝΥυ τΒΡΈο÷ άύ±πΓΘ
―άΗύ | ΝΫΟφ’κΕυΆ·―άΗύ | ’δ÷ιΆθΖά≥τ―άΗύ | ÷–ΜΣΆΗΟς―άΗύ |
ΡΠ≤ΝΦΝ | «β―θΜ·¬Ν | ΧΦΥαΗΤ | Εΰ―θΜ·Ιη |
ΡΠ≤ΝΦΝΒΡΈο÷ άύ±π(÷ΗΥαΓΔΦνΓΔ―ΈΓΔ―θΜ·ΈοΓΔΝΫ–‘«β―θΜ·Έο) |
|
|
|
(2)ΗυΨίΡψΒΡΆΤ≤βΘ§―άΗύΡΠ≤ΝΦΝΒΡ»ήΫβ–‘ «________________________
(ΧνΓΑ“Ή»ήΓ±ΜρΓΑΡ―»ήΓ±)ΓΘ
(3)―άΗύ÷–ΒΡΡΠ≤ΝΦΝΧΦΥαΗΤΩ…“‘”Ο ·Μ“ ·ά¥÷Τ±ΗΘ§Ρ≥―ß…ζ…ηΦΤΝΥ“Μ÷÷ Β―ι “÷Τ±ΗΧΦΥαΗΤΒΡ Β―ιΖΫΑΗΘ§ΤδΝς≥ΧΆΦΈΣΘΚ
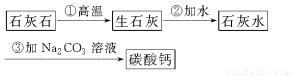
«κ–¥≥ω…œ ωΖΫΑΗ÷–”–ΙΊΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ§≤ΔΉΔΟςΖ¥”Πάύ–ΆΘΚ
ΔΌ________________________________________________ΘΜ
ΔΎ________________________________________________ΘΜ
Δέ________________________________________________ΓΘ
(4)«κΡψ»‘”Ο ·Μ“ ·Ής‘≠Νœ(ΤδΥϊ ‘ΦΝΉ‘―Γ)Θ§…ηΦΤ Β―ι “÷Τ±ΗΧΦΥαΗΤΒΡΝμ“Μ÷÷ Β―ιΖΫΑΗΘ§“ά’’(3)Υυ ΨΘ§ΫΪΡψΒΡ Β―ιΖΫΑΗ”ΟΝς≥ΧΆΦ±μ Ψ≥ωά¥ΘΚ
·Μ“ ·®DΓζ
Ρψ…ηΦΤΒΡΖΫΑΗΒΡ”≈ΒψΈΣΘΚ________________________________ΓΘ
(5)Φλ―ι―άΗύ÷– «ΖώΚ§”–ΧΦΥαΗΤΒΡ Β―ιΖΫΖ® «ΘΚ__________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ1ΝΖΈο÷ Ζ÷άύΜ·―ß”Ο”οΚΆ≥Θ”ΟΦΤΝΩΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
…ηnAΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ ΐ÷ΒΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
(ΓΓΓΓ)
AΘ°≥ΘΈ¬≥Θ―Ιœ¬Θ§8 g O2Κ§”–4nAΗωΒγΉ”
BΘ°1 L 0.1 molΓΛLΘ≠1ΒΡΑ±Υ°÷–”–nAΗωNH4+
CΘ°±ξΉΦΉ¥Ωωœ¬Θ§22.4 L―ΈΥαΚ§”–nAΗωHClΖ÷Ή”
DΘ°1 mol Na±ΜΆξ»Ϊ―θΜ·…ζ≥…Na2O2Θ§ ß»Ξ2nAΗωΒγΉ”
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ15ΝΖ”–ΜζΜ·ΚœΈοΒΡΆΤΕœ”κΚœ≥…ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
œ¬Ν– «“‘ΖΦœψΧΰAΈΣ‘≠Νœ÷Τ±Η±βΧ“Υα( )ΒΡΝς≥ΧΆΦΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
)ΒΡΝς≥ΧΆΦΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ

(1)AΒΡΫαΙΙΦρ ΫΈΣ________Θ§D÷–ΙΌΡήΆ≈ΒΡΟϊ≥Τ «________ΓΘ
(2)C÷–Υυ”–ΧΦ‘≠Ή”________(ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±)Ι≤ΟφΘ§≤ζΈοBΒΡΫαΙΙΦρ ΫΩ…Ρή”–ΝΫ÷÷Θ§Ζ÷±πΈΣ________ΓΘ
(3)–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
EΓζFΘΚ________________________________________________________ΘΜ
GΓζHΘΚ_______________________________________________________ΓΘ
(4)…œ ωΉΣΜΜ÷– τ”ΎΦ”≥…Ζ¥”ΠΒΡ «________(Χν–ρΚ≈)ΓΘ
(5)…ηΦΤ Β―ι÷ΛΟςΈο÷ BΚ§”–¬»‘ΣΥΊ________________________________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ13ΝΖΈο÷ ΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
AΓΔBΓΔCΓΔD «‘ΣΥΊ÷ήΤΎ±μ÷–«Α36Κ≈‘ΣΥΊΘ§ΥϋΟ«ΒΡΚΥΒγΚ… ΐ“ά¥Έ‘ω¥σΓΘΒΎΕΰ÷ήΤΎ‘ΣΥΊA‘≠Ή”ΒΡΚΥΆβ≥…Ε‘ΒγΉ” ΐ «Έ¥≥…Ε‘ΒγΉ” ΐΒΡ2±Ε«“”–3ΗωΡήΦΕΘ§B‘≠Ή”ΒΡΉνΆβ≤ψpΙλΒάΒΡΒγΉ”ΈΣΑκ≥δ¬ζΫαΙΙΘ§C «ΒΊΩ«÷–Κ§ΝΩΉνΕύΒΡ‘ΣΥΊΓΘD «ΒΎΥΡ÷ήΤΎ‘ΣΥΊΘ§Τδ‘≠Ή”ΚΥΆβΉνΆβ≤ψΒγΉ” ΐ”κ«β‘≠Ή”œύΆ§Θ§Τδ”ύΗς≤ψΒγΉ”Ψυ≥δ¬ζΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)AΓΔBΓΔCΒΡΒΎ“ΜΒγάκΡή”…–ΓΒΫ¥σΒΡΥ≥–ρ «________________________(”ΟΕ‘”ΠΒΡ‘ΣΥΊΖϊΚ≈±μ Ψ)ΘΜΜυΧ§D‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ
______________________________________________________________ΓΘ
(2)AΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΖ÷Ή”÷–Θ§Τδ÷––Ρ‘≠Ή”≤…»Γ________‘”Μ·ΘΜBC3ΓΣΒΡΩ’ΦδΙΙ–ΆΈΣ________(”ΟΈΡΉ÷Οη ω)ΓΘ
(3)1 mol ABΘ≠÷–Κ§”–ΒΡΠ–ΦϋΗω ΐΈΣ________ΓΘ
(4)»γΆΦ «Ϋπ τCaΚΆDΥυ–Έ≥…ΒΡΡ≥÷÷ΚœΫπΒΡΨßΑϊΫαΙΙ Ψ“βΆΦΘ§‘ρΗΟΚœΫπ÷–CaΚΆDΒΡ‘≠Ή”Ηω ΐ±» «________ΓΘ

(5)ογΡχΚœΫπ”κ…œ ωΚœΫπΕΦΨΏ”–œύΆ§άύ–ΆΒΡΨßΑϊΫαΙΙXYnΘ§ΥϋΟ«”–Κή«ΩΒΡ¥Δ«βΡήΝΠΓΘ“―÷ΣογΡχΚœΫπLaNinΨßΑϊΧεΜΐΈΣ9.0ΓΝ10Θ≠23cm3Θ§¥Δ«βΚσ–Έ≥…LaNinH4.5ΚœΫπ(«βΫχ»κΨßΑϊΩ’œΕΘ§ΧεΜΐ≤Μ±δ)Θ§‘ρLaNin÷–nΘΫ________(Χν ΐ÷Β)ΘΜ«β‘ΎΚœΫπ÷–ΒΡΟήΕ»ΈΣ________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΩΦΜ·―ß÷ΗΒΦ≥εΙΊ ΒΎ10ΝΖ≥ΘΦϊ”–ΜζΜ·ΚœΈοΦΑΤδ”Π”ΟΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–Ζ¥”Π÷–Θ§ τ”Ύ»Γ¥ζΖ¥”ΠΒΡ «(ΓΓΓΓ)
ΔΌCH3CH=CH2ΘΪBr2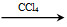 CH3CHBrCH2Br
CH3CHBrCH2Br
ΔΎCH3CH2OH CH2=CH2ΓϋΘΪH2O
CH2=CH2ΓϋΘΪH2O
ΔέCH3COOHΘΪCH3CH2OH CH3COOCH2CH3ΘΪH2O
CH3COOCH2CH3ΘΪH2O
ΔήC6H6ΘΪHNO3 C6H5NO2ΘΪH2O
C6H5NO2ΘΪH2O
AΘ°ΔΌΔΎΓΓΓΓΓΓΓΓΓΓΓΓBΘ°ΔέΔή
CΘ°ΔΌΔέ DΘ°ΔΎΔή
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com