
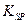 =2.6��10-39��Mg(OH)2���ܶȻ�����
=2.6��10-39��Mg(OH)2���ܶȻ�����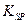 =5.6��
=5.6��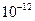 ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��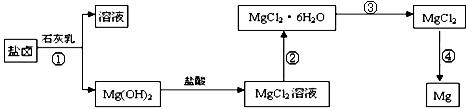
 .���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl�������� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�0.1mol��L-1��CH3COONa��Һ��pH=8 |
| B�������£�0.1mol��L-1��CH3COOH��Һ��pH="3" |
| C�������м�ˮ����Һ��pH���� |
| D��0.1mol��L-1��CH3COOH������������0.1mol��L-1������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���գ�
���գ� CH3COOH��Һϡ��100��������
CH3COOH��Һϡ��100�������� ��ҺpH a +2���>����<����
��ҺpH a +2���>����<���� ��
�� �����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ��
�����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͬ�¡�ͬŨ�ȵ�������ҺPH��NaA��NaB |
| B��PH��ͬ��HA��HB������Һ��ϡ����ͬ������PH��HA��HB |
| C���к͵��������PH��HA��HB��Һʱ��HA����NaOH�����ʵ����� |
| D��ͬ�¡�ͬŨ�ȵ���������Һ��HA�ĵ���̶ȴ���HB�ĵ���̶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��NaHS ����ˮ:��NaHS ==Na+ + HS- HS-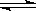 H+ + S2- H+ + S2- |
B��(NH4)2SO4����ˮ�� (NH4)2SO4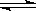 2NH4+ + SO42- 2NH4+ + SO42- |
| C����������ˮ��:��H3PO4 ="=" 3H+ + PO43- |
| D��Al(OH)3�ĵ���:��Al(OH)3 = Al3+ + 3OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaHCO3������Na++HCO3�� | B��NaHSO4������Na++H++SO42�� |
C�� H2CO3����2H++CO32������ H2CO3����2H++CO32������ | D��KCl O3����K++ClO3- O3����K++ClO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������ | B�������� | C�������������С | D������С���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaHCO3=Na++H++CO32- | B��Fe2(SO4)3 = Fe3+ + SO42- |
| C��KClO3=K++Cl��+3O2- | D��H2SO4 = 2H++SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ�����ᷢ���кͷ�Ӧ | B����ˮ��ʹ��ɫʯ����Һ���� |
| C��0.1 mol/L��NH4Cl��Һ��pHֵΪ5.1 | D��Ũ��ˮ�ӷ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com