
 CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1 CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1
CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1 CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0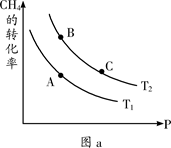
 CO2(g) + H2(g) ��H=��41��2 kJ��mol��1 ��2�֣�
CO2(g) + H2(g) ��H=��41��2 kJ��mol��1 ��2�֣� CO2(g) + H2(g) ��H=��41��2 kJ��mol��1��
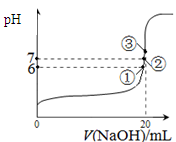
CO2(g) + H2(g) ��H=��41��2 kJ��mol��1��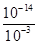 ��10-11mol��L��1��pH��11��NaF��Һ�У���ˮ�������c(H+)��10-14/10-11��10-3mol��L��1��A�����ٵ�ʱ�����ݵ����غ㣬c(OH��)��c(F��)��c(Na+)��c(H��)��c(F��)��c(Na+)��c(H��)��c(OH��)��10-6��
��10-11mol��L��1��pH��11��NaF��Һ�У���ˮ�������c(H+)��10-14/10-11��10-3mol��L��1��A�����ٵ�ʱ�����ݵ����غ㣬c(OH��)��c(F��)��c(Na+)��c(H��)��c(F��)��c(Na+)��c(H��)��c(OH��)��10-6��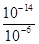 ��9��9��10-7mol/L��B�ԣ����ݵ����غ㣬c(OH��)��c(F��)��c(Na+)��c(H��)���ڵ�ʱpH=7����Һ�е�c(H��)��c(OH��)������c(F��)��c(Na+)��C�ԣ��۵�ʱV��20mL����ʱ��Һ��c(Na+)��
��9��9��10-7mol/L��B�ԣ����ݵ����غ㣬c(OH��)��c(F��)��c(Na+)��c(H��)���ڵ�ʱpH=7����Һ�е�c(H��)��c(OH��)������c(F��)��c(Na+)��C�ԣ��۵�ʱV��20mL����ʱ��Һ��c(Na+)��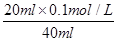 ��0��05mol/L������ѡBC��
��0��05mol/L������ѡBC��

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���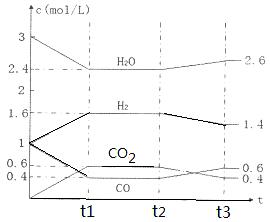
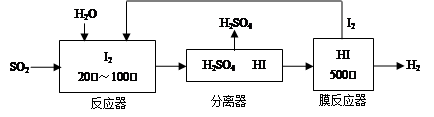
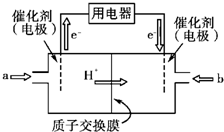
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | Si��O | Si��Cl | H��H | H��Cl | Si��Si | Si��C |
| ����/kJ��mol-1 | 460 | 360 | 436 | 431 | 176 | 347 |
 ����ʾ�辧���е�һ��ԭ��,����������Ķ����á�
����ʾ�辧���е�һ��ԭ��,����������Ķ����á� ����ʾ����֮���ڵĹ�ԭ�ӡ�
����ʾ����֮���ڵĹ�ԭ�ӡ�
 Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol��
Si(s)+4HCl(g),�÷�Ӧ�ķ�Ӧ�Ȧ�H=������kJ/mol�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
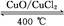 2Cl2+2H2O
2Cl2+2H2O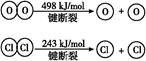
| A����ӦA�Ħ�H>-115.6 kJ/mol |
| B���Ͽ�1 mol H��O����Ͽ�1 mol H��Cl�������������ԼΪ32 kJ |
| C��H2O��H��O����HCl��H��Cl���� |
| D���ɢ��е������ж���Ԫ�صķǽ����Ա���Ԫ��ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-1641.0kJ/mol | B��+3081kJ/mol |
| C��+663.5kJ/mol | D��-2507.0kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��| A�������¶� | B������̼������ | C��������� | D����CO���ռ���ȥCO |
 2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g)
2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g) CO2(g)��H2(g)���ʱ䦤H��________��
CO2(g)��H2(g)���ʱ䦤H��________�� CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________��
CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________�� CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�| �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | | |
| 900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
 2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��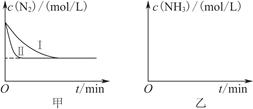
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
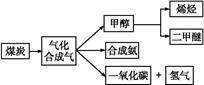
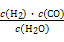 ,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:  CH3OH(g)
CH3OH(g) CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)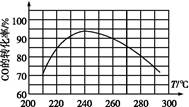
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ?H=bkJ/mol���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ���£�
2NH3(g) ?H=bkJ/mol���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ���£�| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.86 | 0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) ��H <0��
CH3OH(g) ��H <0��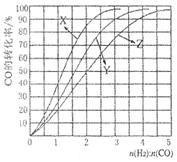
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com