
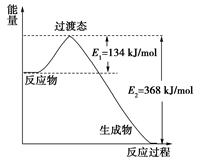
 =4.48L
=4.48L =1mol/L
=1mol/L

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������




 ,�÷�Ӧ���ʱ�
,�÷�Ӧ���ʱ� =________kJ/mol (�ú�a��b��ʽ�ӱ�ʾ)��
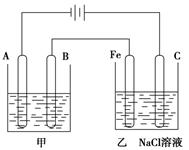
=________kJ/mol (�ú�a��b��ʽ�ӱ�ʾ)�� �������ڴ����������ṩ���Ӻ͵��ӡ���ͼΪ�õ�ص�ʾ��ͼ����缫d�Ϸ����ĵ缫��ӦʽΪ________________��
�������ڴ����������ṩ���Ӻ͵��ӡ���ͼΪ�õ�ص�ʾ��ͼ����缫d�Ϸ����ĵ缫��ӦʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O��SiO2�ۻ� | B��S��Mg�ۻ� |
| C����ɱ����� | D���Ȼ��ƺ������ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2��Br2��Ӧ���Ȼ�ѧ����ʽΪ��H2(g)��Br2(g)===2HBr(g)����H����102kJ |
| B��2 L HBr(g)�ֽ��1 L H2(g)��1 L Br2(g)����102 kJ������ |
| C��1 mol H2(g)��1 mol Br2(l)��Ӧ����2 mol HBr(g)�ų�����������102 kJ |
| D������ͬ�����£�1 mol H2(g)��1 mol Br2(g)�������ܺʹ���2 mol HBr(g)������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����̼��ȼ�����á�H3����ʾ�����H3<��H1 |
| B����̼��ȼ�����á�H3����ʾ�����H3>��H1�� |
| C��Ũ������ϡNaOH��Һ��Ӧ���к���Ϊ57.3kJ/mol |
| D��ϡ������ϡNaOH��Һ��Ӧ����1molˮ���ų�57.3kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��ʾ����8molS�γ�8mol S��S����֪ƽ��1molS���е�S��S��Ȼ�����)����Q��_________��
��ʾ����8molS�γ�8mol S��S����֪ƽ��1molS���е�S��S��Ȼ�����)����Q��_________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CH3OH(g) ��H��+37 kJ��mol��1
2CH3OH(g) ��H��+37 kJ��mol��1 3H2(g) + CO2(g) ��H��+49 kJ��mol��1
3H2(g) + CO2(g) ��H��+49 kJ��mol��1 CO(g) + H2O(g) ��H��+41.3 kJ��mol��1
CO(g) + H2O(g) ��H��+41.3 kJ��mol��1 ����ͬ��3���ܱ������У�����ͬ��ʽ
����ͬ��3���ܱ������У�����ͬ��ʽ Ͷ�뷴Ӧ����ֺ��¡���ѹ��������Ӧ�٣���÷�Ӧ�ﵽƽ��ʱ���й��������¡�
Ͷ�뷴Ӧ����ֺ��¡���ѹ��������Ӧ�٣���÷�Ӧ�ﵽƽ��ʱ���й��������¡�| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol CH3OCH3��1mol H2O | 2mol CH3OH | 1mol CH3OH |
| CH3OH��Ũ�ȣ�mol/L�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | ����a kJ | �ų�b kJ | �ų�c kJ |
| ƽ��ʱ�����L�� | V1 | V2 | V3 |
| ��Ӧ��ת���� | �� 1 | �� 2 | �� 3 |
 C. V1 > V3 D. c1=
C. V1 > V3 D. c1= 2c3
2c3 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 2Fe3O2(s)+CO2(g) ��H=��47kJ/mol
2Fe3O2(s)+CO2(g) ��H=��47kJ/mol 2Fe(s)+3CO2(g) ��H=��25kJ/mol
2Fe(s)+3CO2(g) ��H=��25kJ/mol 3FeO(s)+CO2(g) ��H=+19kJ/mol
3FeO(s)+CO2(g) ��H=+19kJ/mol Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ��
Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com