
| Al��OH��3 | ��ʼ���� | ������ȫ | ������ʼ�ܽ� | �����ܽ���ȫ |
| pH | 3.3 | 5.0 | 7.8 | 12.8 |
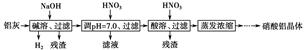
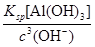 ��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3��
��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3��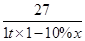 ��
��


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��þ���л����������ۣ���������ռ���Һ��ַ�Ӧ�����ˡ�ϴ�ӡ����� |
| B���ù�����ˮ��ȥFe3����Һ�е�����Al3�� |
| C�������Ƶ���ʯ�ң�ͨ�����������Գ�ȥ�Ҵ��е�����ˮ |
| D��Al(OH)3�л�������Mg(OH)2�����������ռ���Һ����ַ�Ӧ�����ˣ�����Һ��ͨ�����CO2����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(1)(2)(3)(4) | B��(1)(2)(4)(3) | C��(2)(3)(4)(1) | D��(3)(1)(4)(2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
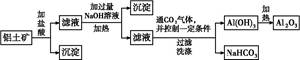
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
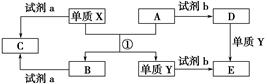
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
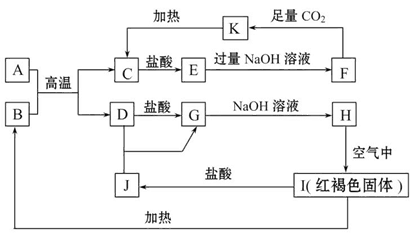
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
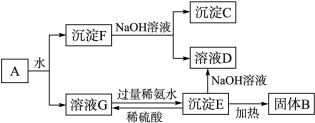
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
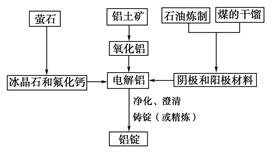
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com