
�±��е��������ƻ�1 mol�����еĻ�ѧ�������ĵ�����(kJ)��
| ���� | H2(g) | O2(g) | H2O(g) |
| ���� | 436 | 496 | 926 |
(1)��Ӧ2H2(g)��O2(g)===2H2O(g)��________(����ȡ����ȡ�)��Ӧ����˵��2 mol H2(g)��1 mol O2(g)���е�������2 mol H2O(g)���е�����________(��ߡ��͡�)��
(2)����ͼʾ��ʾ��2 mol H2(g)��1 mol O2(g)����2 mol H2O(g)�ķ�Ӧ���̣�

(3)���ݱ������ݣ�д��H2(g)��O2(g)��ȫ��Ӧ����H2O(g)���Ȼ�ѧ����ʽ��________________________________________________________________________��
(4)����֪��H2O(g)===H2O(l)������H����44 kJ·mol��1��д��H2(g)��O2(g)��ȫ��Ӧ����H2O(l)���Ȼ�ѧ����ʽ______________________��
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����0.78 mo l��FeCl2��Һ��ͨ��0.009molCl2���ټ��뺬0.01molX2O72-��������Һ��ʹ��Һ�е�Fe2+ǡ��ȫ����������ʹX2O72-��ԭΪXn+����n��ֵ�ǣ� ��
A��2 B��3 C��4 D��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����;�㷺����������(��Ҫ�ɷ�ΪAl2O3·nH2O������SiO2��Fe2O3)��ȡAl������;����
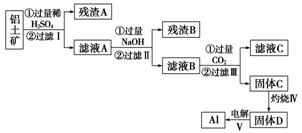
(1)��ҺA�����ھ�ˮ���侻ˮԭ�������ӷ���ʽ��ʾΪ________________________��
(2)����ʱʢ��ҩƷ������������__________��
(3)������з�����Ӧ�Ļ�ѧ����ʽ��____________________________________��
(4)����������ɹ���C��Ӧ�����ӷ���ʽΪ______________________________��
(5)ȡ��ҺB 100 mL������1 mol·L��1����200 mL���������ﵽ���������Ϊ11.7 g������ҺB��c(AlO )��______��c(Na��)______(�>������������<��)2 mol·L��1��
)��______��c(Na��)______(�>������������<��)2 mol·L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���֪ij��Һ����ˮ�������ɵ�H����OH�������ʵ���Ũ�ȵij˻�Ϊ
10��24 mol2��L��2�����ڸ���Һ�У�һ�����ܴ������ڵ�������( )
A��SO32�� B��NH4�� C��NO3�� D��HCO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� �д�ʩ��ˮ�ĵ�����Ӱ�����( )
�д�ʩ��ˮ�ĵ�����Ӱ�����( )
A.�����¶� B.����ϡ����
C.������������ D.����ʳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ѱ�������Դһֱ�ǻ�ѧ��Ŭ���ķ������й�����Դ��˵���д������(����)
A������ȼ���ȸߣ���ȼ�ղ�����ˮ����һ����������ȼ��
B������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ����������
C��ú������������һ���̶���ʵ����ú�ĸ�Ч���������
D��ʯ����Ϊ��Ҫ�Ŀ�������ԴӦ�ñ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ A��B����C(��H��0)���������У���A��B����X (��H ��0)����X����C(��H��0)������ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����(����)
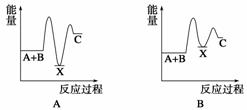
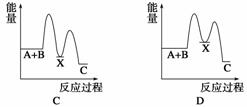
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��HCN(aq)��NaOH(aq)��Ӧ�Ħ�H����12.1 kJ·mol��1��HCl(aq)��NaOH(aq)��Ӧ�Ħ�H����55.6 kJ·mol��1����HCN��ˮ��Һ�е���Ħ�H����(����)
A����67.7 kJ·mol��1 B����43.5 kJ·mol��1
C����43.5 kJ·mol��1 D����67.7 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ϩ��һ��ʳ�����ϣ���ṹ��ʽ��ͼ���й�����ϩ�ķ�����������
A����һ�������£�1mol����ϩ����2molH2��ȫ�ӳ�
B������ϩ��һ�ȴ�����7��
C����һ�������£�����ϩ�ɷ����ӳɡ�ȡ������������ԭ��Ӧ
D������ϩ����������̼ԭ�Ӳ����ܶ���ͬһƽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com