
�ҹ��涨����ˮ�����������������Ҫ��
| pH | 6.5��8.5 |
| Ca2+��Mg2+ | <0.004 5 mol��L-1 |
| ϸ������ | <100��/mL |

��1��Ca2++HCO-3+OH-=CaCO3��+H2O,Mg2++2OH-=Mg��OH��2��?
��2���� Fe��OH��3? ��3������pH ��ȥCa2+?
��4��ɱ������ ǿ����? ��5���٢�?
���������������1��Դˮ�д���Ca2+,Mg2+,HCO-3,Cl-,����ΪCa��HCO3��2,Mg��HCO3��2,CaCl2,MgCl2���ֱ���Ca��OH��2�������ֽⷴӦ����ѧ����ʽΪ��?Ca��HCO3��2+Ca��OH��2=2CaCO3��+2H2O;?Mg��HCO3��2+2Ca��OH��2=Mg��OH��2��+2CaCO3��+2H2O;?MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2?����������������Ӧ����д�����ӷ���ʽ��?Ca2++HCO-3+OH-=CaCO3��+H2O,?Mg2++2HCO-3+2Ca2++4OH-=Mg��OH��2��+2CaCO3��+2H2O,Mg2++2OH-=Mg��OH��2����?
��2������������FeCl3��Һ��Դˮ�����ۼ�������Al3+��Fe3+ˮ���Al��OH��3��Fe��OH��3��������ˮ�������Ĺ����������һ���̼��л�ѧ�仯���������仯�������FeSO4��7H2O�����ۼ���Fe2+����ˮ�⣺Fe2++2H2O Fe��OH��2+2H+,Fe��OH��2��Ѹ�ٱ�������Fe��OH��3:4Fe��OH��2+O2+2H2O=4Fe��OH��3,��������Fe��OH��3������
Fe��OH��2+2H+,Fe��OH��2��Ѹ�ٱ�������Fe��OH��3:4Fe��OH��2+O2+2H2O=4Fe��OH��3,��������Fe��OH��3������
��3��ͨ��CO2��Ŀ���ǵ���pH�ͽ�һ����ȥˮ�е�Ca2+��?
��4��ͨ������A���ڶ�Դˮɱ������������A������ǿ�����ԡ�??
��5������A��������ʱ�����ǿ�����ԣ���ѡ��Ca��ClO��2,K2FeO4,O3�ȡ�
���㣺����ˮ�Ĺ�������
������������������Դˮ����������ˮ�Ĺ������̣��ѶȲ����ؿ���ѧ��ʵ��������������ƶ�����������Ĺؼ���Ҫ��������Ϣ������ÿһ���̵����ã��Ӷ�ȷ����


 �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| pHֵ | 6.5-8.5 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| PH | 6.5��8.5 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PH | 6.5��8.5 |
Ca2+��Mg2+��Ũ��/mol��L-1 | ��0.0045 |
ϸ������/����mol | ��100�� |
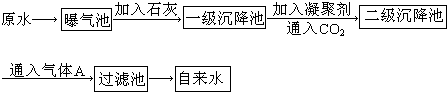
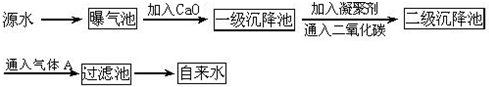
ͼ3-14��ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��
![]()
ͼ3-14
(1)ԭˮ�к�Ca2+��Mg2+��![]() ��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ__________________________________________________��
��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ__________________________________________________��
(2)���ۼ���ȥ������������Ĺ���___________________________��(��д��ţ���ѡ����)
��ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ����
FeSO4��7H2O�dz��õ����ۼ�������ˮ����������___________________________������
(3)ͨ�������̼��Ŀ����__________________��____________________��
(4)����A��������___________�����������ǻ�������A��ˮ��Ӧ�IJ������________�ԡ�
(5)���������У�______________������Ϊ����A�Ĵ���Ʒ��(��д��ţ���ѡ����)
��Ca(ClO)2 ��Ũ��ˮ ��K2FeO4 ��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��涨����ˮ�����������������Ҫ��pHΪ6.5��8.5��Ca2+��Mg2+��Ũ��С��0.004 5 mol��L-1��ϸ������С��100��ÿ��������ͼ��Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к�Ca2+��Mg2+��![]() ��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2����������������ֽⷴӦ����д������������Ӧ�����ӷ���ʽ����__________________����__________________����_________________________________��
��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2����������������ֽⷴӦ����д������������Ӧ�����ӷ���ʽ����__________________����__________________����_________________________________��
(2)���ۼ���ȥ������������Ĺ�����______________________ (�����)��
A.ֻ���������� B.ֻ�ǻ�ѧ���� C.�������ͻ�ѧ����
(3)�̷���ˮ����������______________________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡЭ�������5�µڶ���������ѧ�Ծ��������棩 ���ͣ������
��1�����й��ڹ�ҵ����˵����ȷ���� ��������ţ�
A���ں����Ƽҵ�У����Ȼ�����Һ����ͨ������̼����ͨ����
B�������Ṥҵ���ϳɰ���ҵ�����Ṥҵ�У��Բ���ѭ���������ԭ��������
C�����ȼҵ�����۱����ӽ���Ĥ���������Һ�������
D����ҵ�ϲ��õ�������Ȼ����ķ�����ȡ������
E��ʯ���ѻ����ڻ�ѧ�仯����ҪĿ����Ϊ�˻�ö�����������̬��
��2���ҹ��涨����ˮ�������涨��������±���Ҫ��
|
pH |
Ca2+ ��Mg2+��Ũ�� |
ϸ������ |
|
6.5��8.5 |
�� 0.004 5 mol��L-1? |
��100����mL-1? |
������ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��

��ԭˮ�к�Ca2+ ��Mg2+ ��HCO3- ��Cl-�ȣ�����ʯ������Ca(OH)2�������������ɸ��ֽⷴӦ��д�����е����ӷ���ʽ��ֻҪ��д���������� �� ��
��FeSO4��7H2O�dz��õ����ۼ�������ˮ���������� ������ͨ�������̼��Ŀ���� �� ��
������A�������� ������������ ������Ϊ����A�Ĵ���Ʒ�����ţ���
a��ClO2 b��Ũ��ˮ c��K2FeO4 d��SO2 e.Ư��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com