
������±�����Ϣ���ش��й����⣺
��� |
��Ӧ�¶� ���棩 | �μӷ�Ӧ������ | ||||
Na2S2O3 | H2SO4 | H2O | ||||
V/ml | c/mol?L- | V/ml | c/mol?L- | V/ml | ||
A | 10 | 5 | 0.1 | 5 | 0.1 | 5 |
B | 10 | 5 | 0.1 | 5 | 0.1 | 10 |
C | 30 | 5 | 0.1 | 5 | 0.1 | 10 |
D | 30 | 5 | 0.2 | 5 | 0.2 | 10 |
��1��ʵ��ȽϷ��������о��������ⳣ�õķ��������ڱȽ�ijһ���ض�ʵ�������Ӱ��ʱ�������ų��������صı䶯���ţ�����Ҫ���ƺ���ʵ���йصĸ��Ӧ������
�������п��ԱȽϵ������ ��
��2��������Ӧ�����ӷ���ʽΪ
��3��������Ӧ���������� ���������� ��
��4���ж�������Ӧ���ʿ�����ʵ����������� ��
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�� ��� |
��Ӧ�¶� ���棩 |
�μӷ�Ӧ������ | ||||
| Na2S2O3 | H2SO4 | H2O | ||||
| V/mL | c/mol?L-1 | V/mL | c/mol?L-1 | V/mL | ||
| A | 20 | 10 | 0.1 | 10 | 0.1 | 0 |
| B | 20 | 5 | 0.1 | 10 | 0.1 | 5 |
| C | 20 | 10 | 0.1 | 5 | 0.1 | 5 |
| D | 40 | 5 | 0.1 | 10 | 0.1 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
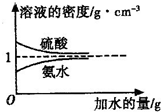 ��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺| ���ʵ����ʵ���Ũ��/mol?L-1 | ��Һ���ܶ�/g?cm-3 | |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
| 1 |
| 5 |
| 3 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
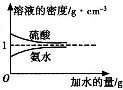 ��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺| ���ʵ����ʵ���Ũ��/mol?L-1 | ��Һ���ܶ�/g?cm-3 | |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
| 1 |
| 5 |
| 3 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
�α���ͨ������ᣨH2C2O4����Һ�еμ��������ữ����������о�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬����д���÷�Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ����ʹ��ָʾ����ԭ���� ��
��ijͬѧ���ݿα���������Ի�ѧ��Ӧ���ʵ�Ӱ��ԭ�����������������������ᷴӦ�й�ʵ�飬ʵ����̵����ݼ�¼���£����������ϱ�����Ϣ���ش��й����⣺
ʵ��
��� ��Ӧ�¶�
���棩 �μӷ�Ӧ������
Na2S2O3 H2SO4 H2O
V/mL c/mol•L-1 V/mL c/mol•L-1 V/mL
A 20 10 0��1 10 0��1 0
B 20 5 0��1 10 0��1 5
C 20 10 0��1 5 0��1 5
D 40 5 0��1 10 0��1 5
��1��д��������Ӧ�����ӷ���ʽ
��2�������������յ�֪ʶ�жϣ�������ʵ���з�Ӧ�������Ŀ����� ����ʵ����ţ�
��3�������ñȽ�ijһ���ض�ʵ�������Ӱ��ʱ�������ų��������صı䶯���ţ�����Ҫ���ƺ���ʵ���� �صĸ��Ӧ���������У�
����˵���¶ȶԸ÷�Ӧ����Ӱ�����ϱȽ��� ������ʵ����ţ�
��A��B��A��C����ϱȽ����о���������
��B��C����ϱȽ����о���������
��4���̲��������˳��ֻ�ɫ�����Ŀ������ȽϷ�Ӧ���ʵĿ������������Ϊ�β�������ˮ��������λʱ������������Ĵ�С�Ŀ���ԭ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com