
����ȡ50mL 0��25 mol/L���ᵹ��С�ձ��У������¶ȣ�
����ȡ50mL 0��55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ���,��Ͼ��Ⱥ�������Һ�¶ȡ�
��ش�
��1��NaOH��Һ�Թ�����ԭ�� ____________��
��2������NaOH��Һ����ȷ������_____________ ������ĸ����
A���ز�������������
B��һ��Ѹ�ټ���
C���������
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__________ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J����g���棩-1��
�����ʵ������д���÷�Ӧ���Ȼ�ѧ����ʽ_____________ ��
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�ϸ߶���2011-2012ѧ��߶���һ���¿���ѧ����(ʵ���) ���ͣ�058
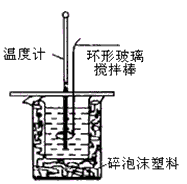
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��50 mL��0.25 mol/L���ᵹ��С�ձ��У����������Һ�¶ȣ�
������һ��Ͳ��ȡ50 mL��0.25 mol/L��NaOH��Һ����������¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȣ�
�ش��������⣺
(1)д��ϡ�����ϡ����������Һ��Ӧ�к��ȵ��Ȼ�ѧ����ʽ(�к���Ϊ��57.3 kJ/mol)��_________��
(2)����NaOH��Һ����ȷ������E(������ѡ��)_________
A���ز�������������
B����������������
C��һ��Ѹ�ٵ���
(3)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������E(������ѡ��)_________
A�����¶ȼ�С�Ľ���
B���ҿ�ӲֽƬ�ò���������
C����������ձ�
D���������¶ȼ��ϵĻ��β���������ؽ���
(4)ʵ���������±���������д�±��еĿհף�
�ڽ�����Ϊ0.25 mol/L��NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18 J��(g����)��1�����к��ȡ�H��_________(ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)_________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c����ȡNaOH��Һ�����ʱ���Ӽ���
d���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ�������и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
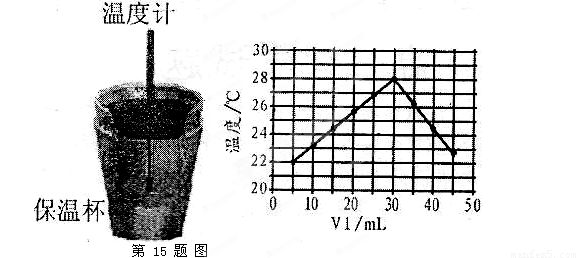
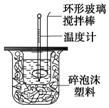
(10��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ�� ��Һ��
��Һ�� δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)��
�ݴ���ش��������⣺ (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
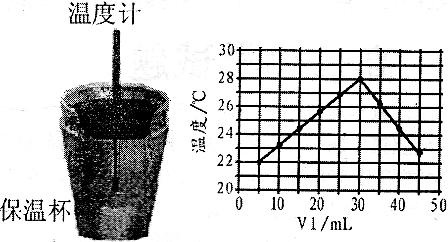
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��1�㼴
��) ____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ��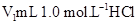 ��Һ��
��Һ��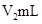 δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)��
�ݴ���ش��������⣺
 (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________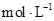
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��1�㼴
��) ____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��������У�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
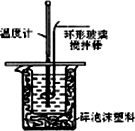
(12��)������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ���
�¶ȡ��ش��������⣺
(1)д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ
57.3 kJ/mol)��_______________________________________________��
(2)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز������������롡 B���������������� C��һ��Ѹ�ٵ���
(3)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
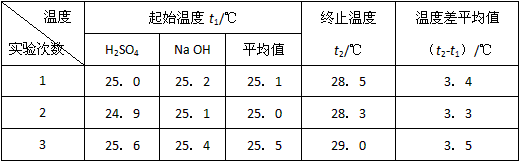
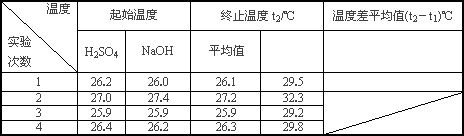
(4)ʵ���������±���
������д�±��еĿհף�
|
�¶� ʵ������� |
��ʼ�¶�t1�� |
��ֹ�¶�t2/�� |
�¶Ȳ�ƽ��ֵ (t2��t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.5 |
|
|
2 |
27.0 |
27.4 |
27.2 |
32.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.2 |
|
|
4 |
26.4 |
26.2 |
26.3 |
29.8 |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��__________ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)__________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ij��ѧѧϰС����ʵ������������ͼװ�òⶨ�кͷ�Ӧ�е���ЧӦ��ʵ��ʱ��![]() ��Һ��
��Һ��![]() δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���![]() )��
)��
�ݴ���ش��������⣺
�� 15 �� ͼ
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
![]() (2)���±���������_______________________________________________________��
(2)���±���������_______________________________________________________��
(3)ijͬѧ�����������ݣ���������¹۵㣬������ȷ����______________________��
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
(4)����ͼ���������ݣ��ɲ��NaOH��Һ��Ũ��ԼΪ______________![]()
(5)����ʹ�ñ��±��⣬Ϊ�˱�֤ʵ��ɹ�����Ҫע����Щ����(˵��2�㼴
��) ____________________________��____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com