

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
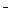 ��OH
��OH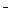 ��SO
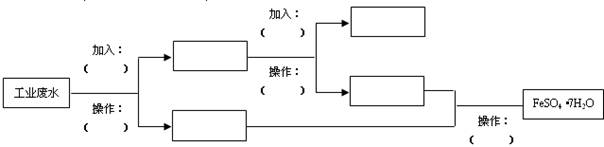
��SO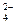 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩��
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������ǿ�����CO2��Ӱ�죩��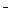
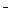 ��
�� L-1H2SO4��0.01mol
L-1H2SO4��0.01mol L-1KMnO4����ɫʯ����Һ����ÿ��2�֣�
L-1KMnO4����ɫʯ����Һ����ÿ��2�֣�| ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ��������Һ���Թ��У��μ�3 moL L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��У� L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��У� | |
| ����2�� | |
| ����3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
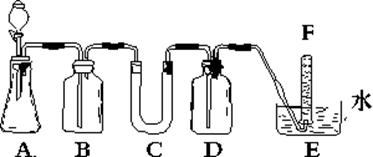
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
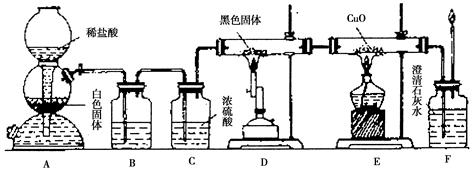
 ��
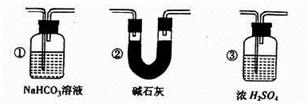
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ô���Һ��ϴ�ζ��õ���ƿ |
| B���к͵ζ�ʱ���ֲ����ζ��ܣ�����ҡ����ƿ���۾�ע�ӵζ���Һ��ı仯 |
| C������ʽ�ζ�����ȡ20.00 mL KMnO4��Һ |
| D��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
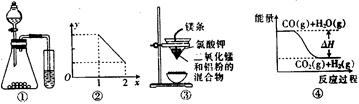
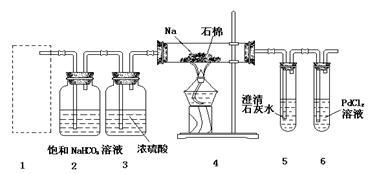
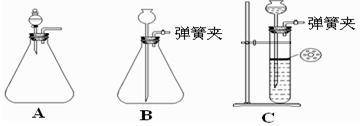
| A����ͼ��ʵ��װ�ÿ���ɱȽ����ᡢ̼�ᡢ������������ǿ����ʵ�� |
| B��ͼ�ڱ�ʾNOx����ˮ��ȫת��ΪHNO3ʱ��x�������y֮��Ĺ�ϵ |
| C����ͼ��װ�ÿ��Ƶý����� |
D��ͼ�ܱ�ʾ���淴ӦCO2 ��g��+H2��g�� CO��g��+H2O��g���ġ�H<O CO��g��+H2O��g���ġ�H<O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ����֤����ȫȼ�պ����ֻ��H2O��CO2 |
| B���ⶨ����������������������ȫȼ�պ�����CO2��H2O������ |
| C���ⶨ��ȫȼ��ʱ�����л��������ɵ�CO2��H2O�����ʵ���֮�� |
| D��ֻ�вⶨ��ȼ�ղ�����H2O��CO2���ʵ����ı�ֵ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com