
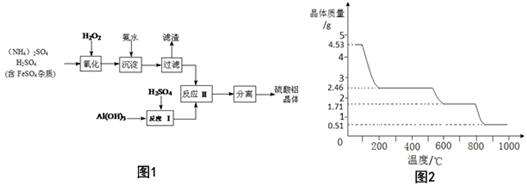
���� �ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˳�ȥ����Һ�к�������泥�����������������Ӧ�õ���������Һ���������Һ����������Һ��Ϻ�Ӧ���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȵõ����������壮
��1�����������£��������⽫��Һ��Fe2+����ΪFe3+��
��2�����˺����Һ�п��ܺ���Fe3+����KSCN��Һ�����Ƿ���Fe3+��
��3�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
��4��4.53g������茶�������ʵ���Ϊ$\frac{4.53g}{906g/mol}$=0.005mol��4.53g������茶�����ˮ������Ϊ0.005mol��24��18g/mol=2.16g������400��ʱ�����������١�m=4.53g-2.46g=2.07g��2.16g��˵��ʧȥ���ֽᾧˮ�����㾧����ʣ��ᾧˮ����������������ʣ�������n[����NH4��2Al2��SO4��4]��n��H2O�����ݴ�ȷ����ѧʽ��
��� �⣺�ɹ������̿�֪������������⽫��Һ��Fe2+����ΪFe3+�����백ˮ������ҺPHֵ��Fe3+ʹת��ΪFe��OH��3�����˳�ȥ����Һ�к�������泥�����������������Ӧ�õ���������Һ���������Һ����������Һ��Ϻ�Ӧ���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȵõ����������壮
��1�����������£��������⽫��Һ��Fe2+����ΪFe3+����Ӧ���ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2�����˺����Һ�п��ܺ���Fe3+�����顰���ˡ��������Ƿ������ʵ�鷽���ǣ�ȡ������Һ���Թ��У��μӼ���KSCN��Һ������Һ�����ɫ���������ѳ���������Һ���ɫ��������δ������
�ʴ�Ϊ��ȡ������Һ���Թ��У��μӼ���KSCN��Һ������Һ�����ɫ���������ѳ���������Һ���ɫ��������δ������
��3�������С����롱�Ǵ���Һ�л�þ��壬����Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȣ�
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��4��4.53g������茶�������ʵ���Ϊ$\frac{4.53g}{906g/mol}$=0.005mol��
4.53g������茶�����ˮ������Ϊ0.005mol��24��18g/mol=2.16g��
����400��ʱ�����������١�m=4.53g-2.46g=2.07g��2.16g��
ʣ������нᾧˮ�����ʵ���Ϊ$\frac{2.16g-2.07g}{18g/mol}$=0.005mol��
ʣ�������n[��NH4��Al��SO4��2]��n��H2O��=0.01mol��0.005mol=2��1��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4•H2O��
��400��ʱʣ�����ɷֵĻ�ѧʽΪ��NH4��2Al2��SO4��4•H2O��
���� ���⿼�������Ʊ��������̡�������ɵļ���ȣ���Ҫѧ���߱���ʵ�Ļ���������֪ʶ��������������������4������ȷ�ж�400��ʱ���յõ��������Ƿ��нᾧˮ�ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3OH��C3H4 | B�� | CH4��C3H4O | C�� | CH4��C2H4 | D�� | CH4��C3H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ⵥ�ʵ����������У�ֻ��˷����Ӽ������� | |
| B�� | NH4Cl�������ӻ������������ֻ�������Ӽ� | |
| C�� | ��N2��CO2��SiO2�����У������ڹ��ۼ������Ƕ����ɷ��ӹ��� | |
| D�� | ���ʯ������ϩ��C60����Ϊԭ�Ӿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
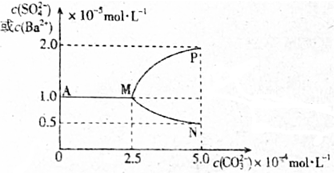
| A�� | T��ʱKsp��BaSO4����Ksp��BaCO3�� | |
| B�� | ��c��CO32-������2.5��10-4mol•L-1ʱ��ʼ��BaCO3�������� | |
| C�� | ͼ���д�������ת��������c��Ba2+����c��CO32-���仯��������MP | |
| D�� | ��ӦBaSO4��s��+CO32-��aq��?BaCO3��s��+SO42-��aq����ƽ�ⳣ��K=0.04 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ӦI2��aq��+I-��aq��?I3-��aq����H��0 | |
| B�� | �¶�ΪT1ʱ�����ƽ����ϵ�м���KI���壬ƽ�ⲻ�ƶ� | |
| C�� | ��T1ʱ����Ӧ���е�״̬dʱ��һ����v����v�� | |
| D�� | ״̬a��״̬b��ȣ�״̬bʱI2��ת���ʸ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com