
 ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g?cm-3��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʣ�
ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g?cm-3��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���㶫ʡ�����а�����ѧ2011������ڶ����¿���ѧ���� ���ͣ�058
ʵ��������Ҫ22.4 L(��״��)SO2���壮��ѧС��ͬѧ���ݻ�ѧ����ʽZn��2H2SO4(Ũ)![]() ZnSO4��SO2����2H2O�����ȡ65.0 gп����98����ŨH2SO4(�ѣ�1.84 g��cm��3)110 mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ�������
ZnSO4��SO2����2H2O�����ȡ65.0 gп����98����ŨH2SO4(�ѣ�1.84 g��cm��3)110 mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ�������
(1)��ѧС�����Ƶõ������л��е���Ҫ�������������________(�����ʽ)���������ֽ������Ҫԭ����________(�û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵��)
(2)Ϊ֤ʵ��ط�������ѧС���ͬѧ�����ʵ�飬��װ������װ�ã�������ȡ���������̽����
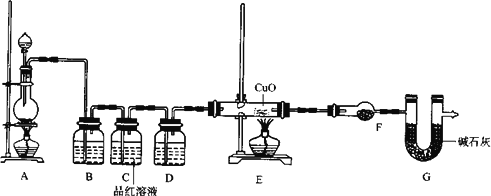
��װ��B�м�����Լ�________��������________��
��װ��D�м�����Լ�________��װ��F�м�����Լ�________��
�ۿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���ij���������ʵ��������________��
��U��G��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ����������ѧ2011�������ѧ����ĩ���Ի�ѧ���� ���ͣ�058
ʵ��������Ҫ22.4 L(��״��)SO2���壮��ѧС��ͬѧ���ݻ�ѧ����ʽZn��2H2SO4(Ũ)![]() ZnSO4��SO2����2H2O�����ȡ65.0 gп����98����ŨH2SO4(�ѣ�1.84 g��cm��3)110 mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ�������
ZnSO4��SO2����2H2O�����ȡ65.0 gп����98����ŨH2SO4(�ѣ�1.84 g��cm��3)110 mL��ַ�Ӧ��пȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ�������
(1)��ѧС��������Ƶõ������л��е���Ҫ�������������________(�����ʽ)��
(2)Ϊ֤ʵ��ط�������ѧС���ͬѧ�����ʵ�飬��װ����װ�ã�������ȡ���������̽����
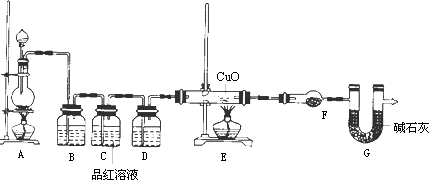
��װ��B�м�����Լ�________��������________��
��װ��D�м�����Լ�________��װ��F�м�����Լ�________��
�ۿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���ij���������ʵ��������________��
��U��G��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� 13�֣�ʵ��������Ҫ22��4 l����״����SO2���塣��ѧС��ͬѧ���ݻ�ѧ����ʽZn+2H2SO4��Ũ��![]() ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��
ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��![]() ��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
��1����ѧС�����Ƶõ������л��е���Ҫ������������� �������ʽ�����������ֽ������Ҫԭ����
���û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵����
��2��Ϊ֤ʵ��ط�������ѧС���ͬѧ�����ʵ�飬��װ������װ�ã�������ȡ���������̽����
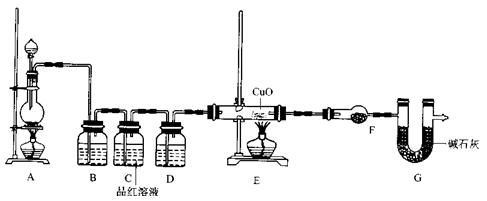
��װ��B�м�����Լ� �������� ��
��װ��D������Լ� ��װ��F������Լ� ��
�ۿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���ij���������ʵ�������� ��
��U��G������Ϊ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ������2010�������һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
�� 13�֣�ʵ��������Ҫ22��4 l����״����SO2���塣��ѧС��ͬѧ���ݻ�ѧ����ʽZn+2H2SO4��Ũ��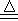 ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��
ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��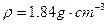 ��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
��1����ѧС�����Ƶõ������л��е���Ҫ������������� �������ʽ�����������ֽ������Ҫԭ����
���û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵����
��2��Ϊ֤ʵ��ط�������ѧС���ͬѧ�����ʵ�飬��װ������װ�ã�������ȡ���������̽����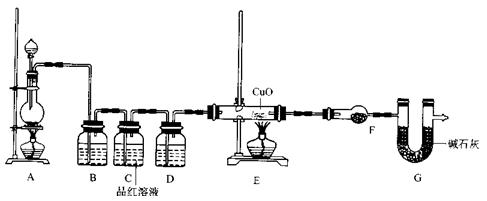
��װ��B�м�����Լ� �������� ��
��װ��D������Լ� ��װ��F������Լ� ��
�ۿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���ij���������ʵ�������� ��
��U��G������Ϊ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ��������Ҫ22��4 l����״����SO2���塣��ѧС��ͬѧ���ݻ�ѧ����ʽZn+
�� 2H2SO4��Ũ����![]() ���� ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��
���� ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4��![]() ��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
��110mL��ַ�Ӧпȫ���ܽ⣬�����Ƶõ����壬��ͬѧ��Ϊ���ܻ������ʡ�
�� ��1����ѧС�����Ƶõ������л��е���Ҫ������������������������� �������ʽ�����������ֽ������Ҫԭ������������������������������������������������
�� ���û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵����
�� ��2��Ϊ֤ʵ��ط�������ѧС���ͬѧ�����ʵ�飬��װ������װ�ã�������ȡ���������̽����
|
��װ��B�м�����Լ��� ������ ���������������������������� ��
��װ��D������Լ��������� ��װ��F������Լ��������������������� ��
�ۿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���ij���������ʵ���������������������������������������������� ��
��U��G�������������������������������������������� .
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com