
| n |
| V |
| 3.0mol/L��0.25L |
| 18mol/L |
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��CH3COO-����c ��Na+�� |
| B��c��Na+��+c��H+��=c��OH-��+c��CH3COOH��+c��CH3COO-�� |
| C��c��CH3COOH����c��CH3COO-�� |
| D��c��CH3COOH��+c��CH3COO-��=0.02mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
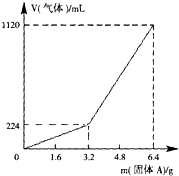 ��һ������Ͼ��ȵ�����������ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300mL 2mol?L-1������ʹ֮��ȫ�ܽ⣮��������¼������A���������ռ�������������ѻ���ɱ�״�����Ĺ�ϵ��ͼ15��ʾ������������������Һ����ǰ�����������ݳ�����
��һ������Ͼ��ȵ�����������ڸ��������������¹��ȣ���ַ�Ӧ����ȴ�����£��õ�����A��������Ϊm�Ĺ���A���뵽300mL 2mol?L-1������ʹ֮��ȫ�ܽ⣮��������¼������A���������ռ�������������ѻ���ɱ�״�����Ĺ�ϵ��ͼ15��ʾ������������������Һ����ǰ�����������ݳ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaClOˮ��Һ�У�Fe2+��Cl-��Ca2+��H+ |
| B������KSCN�Ժ�ɫ����Һ��K+��Na+��I-��S2- |
| C����ɫ������Һ�У�K+��CH3COO-��HCO3-��MnO4- |
| D��pH=2����Һ�У�NH4+��Na+��Cl-��Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe2O3��������Fe2O3+6HI=2Fe3++6I-+3H2O |
| B��Na2S��Һ�Լ��ԣ�S2-+2H2O=H2S+2OH- |
| C��CH3COOH��NaOH��Һ��Ӧ��H++2OH-=H2O |
| D��AgNO3��Һ�м��������ˮ��Ag++2NH3?H2O=Ag��NH3��2++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��46g�Ҵ��к��еĻ�ѧ����Ϊ7NA |
| B��1molOH-��1mol-OH���ǻ����к��е���������Ϊ9NA |
| C��22.4L����������NaOH��Һ��Ӧת�Ƶ�����ΪNA |
| D��0.1mol?L-1FeCl3��Һ�Ƴɽ��壬����Fe��OH��3������һ��С��O.1 NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com