
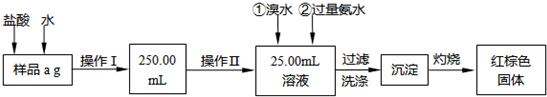
��15�֣�ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

������������̣��ش��������⣺
��1������I���õ��IJ����������ձ�������������Ͳ�⣬��������____________ �����������ƣ�������II�����õ���������____________�����ţ���
A��50mL�ձ��� B��50mL��Ͳ
C��25mL��ʽ�ζ��ܡ� D��25mL��ʽ�ζ���
��2��ϴ����ϴȥ�����ڳ����ϵ�____________����д���ӷ��ţ�
��3����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ�����________________________��
��4������������ȣ������ڸ���������ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3g�����������Ӧ���еIJ�����_________��
��5����������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������____________��
��6����ͬѧ��Ϊ����������������������ˮ���������費�䣬�ԿɴﵽĿ�ġ�����������________________________�����û�ѧ����ʽ��ʾ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 7(W2-W1) |
| a |
| 7(W2-W1) |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 112(W2-W1) |
| 160a |
| 112(W2-W1) |
| 160a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
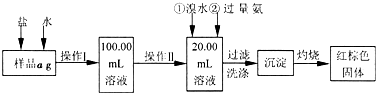
��Ŀ�����л�ѧ ��Դ������ʡ�Ƹ���2009��2010ѧ��ȸ����꼶��ĩ���������ۺ��������Ի�ѧ���� ���ͣ�ʵ����
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
������������̣��ش��������⣺
��1������I���õ��IJ����������ձ�������������Ͳ�⣬��������____________ ������ѡ�������ƣ�������II�����õ���������____________�����ţ���
����
| A��50mL�ձ� | B��50mL��Ͳ ���� | C��25mL��ʽ�ζ��� | D��25mL��ʽ�ζ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и����ڶ���������⻯ѧ�Ծ� ���ͣ������
��15�֣�ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

������������̣��ش��������⣺
��1������I���õ��IJ����������ձ�������������Ͳ�⣬��������____________ �����������ƣ�������II�����õ���������____________�����ţ���
A��50mL�ձ��� B��50mL��Ͳ
C��25mL��ʽ�ζ��ܡ� D��25mL��ʽ�ζ���
��2��ϴ����ϴȥ�����ڳ����ϵ�____________����д���ӷ��ţ�
��3����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ�����________________________��
��4������������ȣ������ڸ���������ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3g�����������Ӧ���еIJ�����_________��
��5����������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������____________��
��6����ͬѧ��Ϊ����������������������ˮ���������費�䣬�ԿɴﵽĿ�ġ�����������________________________�����û�ѧ����ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com