
ij�о���ѧϰС���ѧ����һ�λ�У���Ƴ����·������ⶨͭ�����ԭ��������
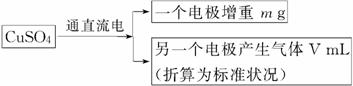
ָ����ʦ��Ϊ�˷������У��ṩ��һƿ����һ����H2SO4��Fe2(SO4)3���ʵ�CuSO4��Һ���������ҩƷ�����ġ�
(1)ͬѧ���������۲�����CuSO4��Һ�Ƿ���Ҫ�ᴿ����������ѧϰС���ѧ�����������Ŀ�����(�����������ֻ��ѡ��a��b��С����һ��������һ�ʲ��ػش�)
a����Ҫ�ᴿ������________(��д�Լ���ѧʽ)����Ӧ���˼����ᴿCuSO4��Һ��
b�������ᴿ��������
________________________________________________________________________��
(2)��ͭ��ʯī�����缫���CuSO4��Һʱ��ͭ�缫Ӧ�ӵ�Դ��________����ʯī�缫�ϵĵ缫��Ӧʽ��________________________________________����һ���ķ�Ӧʽ��
________________________________________________________________________��
(3)��ʵ����ͭ�����ԭ��������
________________________________________________________________________
(�ú�m��V�Ĵ���ʽ��ʾ)��
������(1)���ʻ�Ӱ��CuSO4��Һ�ĵ�⣬��ΪFe3���������Ա�Cu2��ǿ�����ʱ�����ȷŵ磬���Ա����ᴿ�����Լ���CuO��Cu(OH)2��CuCO3�����ʣ�������Һ������ԣ�ʹFe3����Fe(OH)3����ʽ�������������˳�ȥ��
(2)��Ϊ�����ϱ�����Cu2���ŵ磬����ֻͭ����Ϊ������ֻ�����Դ�ĸ���������ʯī��������OH���ŵ硣
(3)�ɸ����ܵ�ⷴӦ����ʽ���м��㡣
�𰸣�(1)a.CuO[��Cu(OH)2��CuCO3]
(2)����4OH����4e��===2H2O��O2����Cu2����2e��===Cu
(3)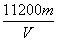


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е� λ�ã�����ѧ�����ش��������⣺
λ�ã�����ѧ�����ش��������⣺
| �� ���� | ��A | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 |
| �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� |
��1����Ԫ���⻯��ĵ���ʽ�� ��
��2���ڡ��ۡ���Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ____________________��
��3���ڡ��ܡ���Ԫ�ص���̬�⻯���ȶ�����ǿ������˳����_________________________��
��4�������ֻ�����A��B���ɢ٢ܢݢ�����Ԫ����ɡ���A��B��ˮ��Һ���ܷ������ӷ�Ӧ����÷�Ӧ�����ӷ���ʽΪ  ��
��
��5 ������Ԫ��������������Ӧ��ˮ�����������ǿ����
������Ԫ��������������Ӧ��ˮ�����������ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¶Ȳ���������£��ܱ������з������·�Ӧ��2SO2��O2 2SO3�����������ܹ�˵����Ӧ�Ѿ��ﵽƽ��״̬����
2SO3�����������ܹ�˵����Ӧ�Ѿ��ﵽƽ��״̬����
A. ������SO2��O2��SO3���� B. SO2��SO3��Ũ�����
C. ������SO2��O2��SO3�����ʵ���֮��Ϊ2��1��2 D. ��Ӧ������ѹǿ����ʱ��仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ����(����)
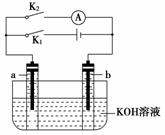 A���Ͽ�K2���պ�K1һ��ʱ�䣬��Һ��pH���
A���Ͽ�K2���պ�K1һ��ʱ�䣬��Һ��pH���
B���Ͽ�K1���պ�K2ʱ��b���ϵĵ缫��ӦΪ2H����2e��===H2��
C���Ͽ�K2���պ�K1ʱ��a���ϵĵ缫��ӦΪ4OH����4e��===O2����2H2O
D���Ͽ�K1���պ�K2ʱ��OH����b���ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��a��b������ʯī��������������ȷ����(����)

A��a��������������ԭ��Ӧ
B��b������������������Ӧ
C��ϡ��������������ӵ����ʵ�������
D������ֽ�ϵμӷ�̪��Һ��a��������ɫ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��95�洿ˮ��pH<7��˵�����ȿɵ���ˮ������
B��pH��3�Ĵ�����Һ��ϡ����10����pH��4
C��0.2 mol��L��1������������ˮ��Ϻ�pH��1
D��pH��3�Ĵ�����Һ��pH��11������������Һ�������Ϻ�pH��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����֪Ũ�ȵ�NaOH��Һ�ⶨijH2SO4��Һ��Ũ�ȣ��ο���ͼ���±���ѡ����ȷѡ��(����)

| ��ƿ ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | �� |
| B | �� | �� | ��̪ | �� |
| C | �� | �� | ���� | �� |
| D | �� | �� | ��̪ | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������̼ԭ��Ϊ���ĵ���������ṹ�����������ε�ƽ��ṹ����������(����)
A��CHCl3ֻ��һ�ֽṹ
B��CH2Cl2ֻ��һ�ֽṹ
C��CH4�ǷǼ��Է���
D��CH4���ĸ��ۼ��ļ����ͼ��ܶ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�±�Ϊϩ����������巢���ӳɷ�Ӧ���������(����ϩΪ��)��
| ϩ������� | ������� |
| (CH3)2C===CHCH3 | 10.4 |
| CH3CH===CH2 | 2.03 |
| CH2===CH2 | 1.00 |
| CH2===CHBr | 0.04 |
���ݱ������ݣ��ܽ�ϩ����������ʱ����Ӧ������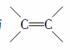 ����ȡ���������ࡢ������Ĺ�ϵ�� _______________________________________
����ȡ���������ࡢ������Ĺ�ϵ�� _______________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������______(�����)��
A��(CH3)2C===C(CH3)2
B��CH3CH===CHCH3
C��CH2===CH2
D��CH2===CHCl
(3)ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
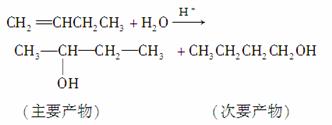
���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ����(�����������Լ�����ȥ)���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�9����Ч��ԭ�ӡ�
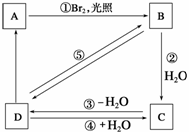
������ͼ�У�B�Ľṹ��ʽΪ________________������ȡ����Ӧ����________(���ͼ�е���ţ���ͬ)��������ȥ��Ӧ����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com