
¢ñ.CuClºÍCuCl2¶¼ÊÇÖØ̉ªµÄ»¯¹¤ÔÁÏ£¬³£ÓĂ×÷´ß»¯¼Á¡¢ÑƠÁÏ¡¢·À¸¯¼ÁºÍÏû¶¾¼ÁµÈ¡£
̉ÑÖª£º¢ÙCuCl¿É̉ÔÓÉCuCl2ÓĂÊʵ±µÄ»¹Ô¼ÁÈçSO2¡¢SnCl2µÈ»¹ÔÖÆµĂ£º
2Cu2£«£«2Cl££«SO2£«2H2O 2CuCl¡ư£«4H£«£«SO42-
2CuCl¡ư£«4H£«£«SO42-
2CuCl2£«SnCl2=2CuCl¡ư£«SnCl4
¢ÚCuCl2ÈÜ̉ºÓë̉̉¶₫°·(H2N¡ªCH2¡ªCH2¡ªNH2)¿ÉĐγÉÅäÀë×Ó£º
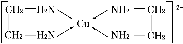
Çë»Ø´đÏÂÁĐÎỀ⣺
(1)»ù̀¬CuÔ×ӵĺËÍâµç×ÓÅŲ¼Ê½Îª____________£»H¡¢N¡¢OÈưÖÖÔªËصĵ縺ĐÔÓÉ´óµ½Đ¡µÄ˳Đ̣ÊÇ_________________________________________________________¡£
(2)SO2·Ö×ӵĿƠ¼ä¹¹ĐÍΪ________£»ÓëSnCl4»¥ÎªµÈµç×Ó̀åµÄ̉»ÖÖÀë×ӵĻ¯Ñ§Ê½Îª________________________________________________________________________¡£
(3)̉̉¶₫°··Ö×ÓÖеªÔ×Ó¹́µÀµÄÔÓ»¯ÀàĐÍΪ________¡£̉̉¶₫°·ºÍÈư¼×°·[N(CH3)3]¾ùÊôÓÚ°·£¬µ«̉̉¶₫°·±ÈÈư¼×°·µÄ·Đµă¸ßµĂ¶à£¬Ộ̉ÊÇ_________________________________¡£
(4)¢ÚÖĐËùĐγɵÄÅäÀë×ÓÖĐº¬ÓеĻ¯Ñ§¼üÀàĐÍÓĐ________(̀î×Öĸ)¡£
a£®Åäλ¼ü ¡¡b£®¼«ĐÔ¼ü ¡¡c£®Àë×Ó¼ü ¡¡d£®·Ç¼«ĐÔ¼ü
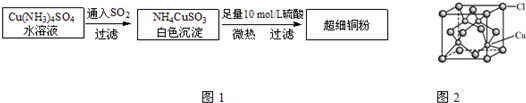
¢̣.¹̀̀å¶₫Ñơ»¯̀¼ÍâĐÎËƱù£¬ÊÜÈÈÆû»¯Î̃̉º̀å²úÉú£¬Ë׳ơ°¸É±ù¡±£¬¸ù¾Ư¸É±ù¾§°û½á¹¹»Ø´đ£º
(5)¸É±ùÖĐ̉»¸ö·Ö×ÓÖÜΧÓĐ________¸ö½ôÁÚ·Ö×Ó¡£
(6)¶Ñ»ư·½Ê½Óë¸É±ù¾§°ûÀàĐÍÏàͬµÄ½đÊôÓĐ________(´Ó¡°Cu¡¢Mg¡¢K¡¢Po¡±ÖĐÑ¡³öƠưÈ·µÄ)£¬Æä¿Ơ¼äÀûÓĂÂÊΪ________¡£
£¨1£©1s22s22p63s23p63d104s1(»̣[Ar]3d104s1)¡¡O£¾N£¾H
(2)VĐΡ¡SO42-¡¢SiO44-µÈ
(3)sp3ÔÓ»¯¡¡̉̉¶₫°··Ö×Ó¼ä¿É̉ÔĐγÉÇâ¼ü£¬Èư¼×°··Ö×Ӽ䲻ÄÜĐγÉÇâ¼ü
(4)abd¡¡(5)12¡¡(6)Cu¡¡74%
¡¾½âÎö¡¿(1)¸ù¾Ưºé̀عæỘ¼°ÄÜÁ¿×îµÍÔÀí¿ÉÖª£¬µ±ÍÔ×ÓµÄ3d¹́µÀÉϵĵç×Ó´¦ÓÚÈ«³äÂú×´̀¬£¬Ơû¸ö̀åϵÄÜÁ¿×îµÍ£¬¼´»ù̀¬CuÔ×ӵĺËÍâµç×ÓʽΪ[Ar]3d104s1¡£ÔªËصķǽđÊôĐÔԽǿ£¬µç¸ºĐÔÔ½´ó£¬·Ç½đÊôĐÔÓÉÇ¿µ½ÈơΪO¡¢N¡¢H¡£(3)̉̉¶₫°·ÖеªÔ×Ó¹́µÀµÄÔÓ»¯ÀàĐ͵ÈͬÓÚ°±·Ö×Ó£¬¸ù¾Ư·Ö×Ó¹¹ĐÍ£¬ËÄĂæ̀åĐÎ(Èư½Ç׶ĐΡ¢VĐÎ)·Ö×ÓΪsp3ÔÓ»¯¡£¸ù¾Ử̉¶₫°·µÄ½á¹¹¼̣ʽ¿ÉÖª̉̉¶₫°··Ö×Ó¼ä´æÔÚÇâ¼ü£¬¶øÈư¼×°··Ö×Ӽ䲻ÄÜĐγÉÇâ¼ü£¬Çâ¼ü¿É̉ÔʹÈÛ¡¢·ĐµăÉư¸ß¡£(4)¸ù¾ƯÅäÀë×ӵĽṹʽ¿ÉÖªÔÚ̉̉¶₫°·ÖĐ´æÔÚ̀¼̀¼Ö®¼äµÄ·Ç¼«ĐÔ¼ü£¬CÓëH£¬NÓëH£¬CÓëNÖ®¼äµÄ¼«ĐÔ¼ü£¬»¹ÓĐNÓëCuÖ®¼äµÄÅäλ¼ü¡£(5)¸É±ù·Ö×Ó¾§̀åµÄ¾§°ûÖĐ£¬CO2·Ö×ÓλÓÚ¾§°ûÁ¢·½̀åµÄ¶¥µăºÍĂæĐÄÉÏ£¬ỘĂ¿¸ö·Ö×ÓÖÜΧ½ôÁÚµÄĂ¿²ăÉÏÓĐ4¸ö£¬3²ă¹²12¸ö¡£



| Ä꼶 | ¸ßÖĐ¿Î³̀ | Ä꼶 | ³ơÖĐ¿Î³̀ |
| ¸ß̉» | ¸ß̉»Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ở» | ³ở»Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ß¶₫ | ¸ß¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ơ¶₫ | ³ơ¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ßÈư | ¸ßÈưĂâ·Ñ¿Î³̀ÍƼö£¡ | ³ơÈư | ³ơÈưĂâ·Ñ¿Î³̀ÍƼö£¡ |
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
| ||


²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º

²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
| ||
| ||
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
 ͵¥Öʼ°Æ仯ºÏÎïÔںܶàÁ́Ọ́ÓĐÖØ̉ªµÄÓĂ;£¬Èç½đÊôÍÓĂÀ´ÖÆỐµçÏßµçÀ£¬³¬Ï¸Í·Û¿ÉÓ¦ÓĂÓÚµ¼µç²ÄÁÏ¡¢´ß»¯¼ÁµÈÁ́Ọ́ÖĐ£¬CuClºÍCuCl2¶¼ÊÇÖØ̉ªµÄ»¯¹¤ÔÁÏ£¬³£ÓĂ×÷´ß»¯¼Á¡¢ÑƠÁÏ¡¢·À¸¯¼ÁºÍÏû¶¾¼ÁµÈ£®
͵¥Öʼ°Æ仯ºÏÎïÔںܶàÁ́Ọ́ÓĐÖØ̉ªµÄÓĂ;£¬Èç½đÊôÍÓĂÀ´ÖÆỐµçÏßµçÀ£¬³¬Ï¸Í·Û¿ÉÓ¦ÓĂÓÚµ¼µç²ÄÁÏ¡¢´ß»¯¼ÁµÈÁ́Ọ́ÖĐ£¬CuClºÍCuCl2¶¼ÊÇÖØ̉ªµÄ»¯¹¤ÔÁÏ£¬³£ÓĂ×÷´ß»¯¼Á¡¢ÑƠÁÏ¡¢·À¸¯¼ÁºÍÏû¶¾¼ÁµÈ£®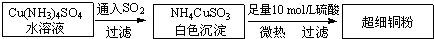
| ||
| ||
²é¿´´đ°¸ºÍ½âÎö>>
°Ù¶ÈÖÂĐÅ - Á·Ï°²áÁбí - ÊỒâÁбí
º₫±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨Æ½̀¨ | ÍøÉÏÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | µçĐÅƠ©Æ¾Ù±¨×¨Çø | ÉæÀúÊ·ĐéÎ̃Ö÷̉åÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com