
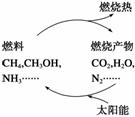
ΧΪ―τΡή ««εΫύΒΡ–¬Ρή‘¥Θ§ΈΣΝΥΜΖ±ΘΘ§Έ“Ο«“ΣΦθ…Ό Ι”ΟœώΟΚΧΩ’β―υΒΡ≥ΘΙφΡή‘¥Εχ¥σΝΠΩΣΖΔ–¬Ρή‘¥ΓΘ
(1)Μ°Ζ÷œ¬Ν–Ρή‘¥ΒΡάύ±πΓΘ
ΟΚΓΔ ·”ΆΓΔΥ°ΝΠΓΔΤϊ”ΆΓΔ”ΥΓΔ–Ϋ≤ώΓΔΨΤΨΪΓΔΧλ»ΜΤχΓΔ“ΚΜ·ΤχΓΔ»»Υ°ΓΔΟΚΤχΓΔ’τΤϊΓΔΖγΝΠΓΔΒγ
| άύ±π | “Μ¥ΈΡή‘¥ | Εΰ¥ΈΡή‘¥ |
| »ΦΝœΡή‘¥ | ||
| Ζ«»ΦΝœΡή‘¥ |
(2)ΟΚΓΔ ·”ΆΓΔΧλ»ΜΤχΚΆ…ζΈο÷ ΡήΉςΈΣΡή‘¥ΒΡΙ≤Ά§ΧΊΒψ «______________Θ§ΡήΝΩ–Έ≥…ΚΆΉΣΜΜάϊ”ΟΙΐ≥ΧΜυ±Ψ…œ «________ΓΘ
(3)ΈΣΝΥœϊ≥ΐ¥σΤχΈέ»ΨΘ§ΫΎ‘Φ»ΦΝœΘ§ΜΚΫβΡή‘¥ΈΘΜζΘ§Μ·―ßΦ“Χα≥ωΝΥάϊ”ΟΧΪ―τΡή¥Ό Ι»ΦΝœ―≠ΜΖ Ι”ΟΒΡΙΙœκΆΦΘ§ΗΟ…ηœκΒΡΈο÷ ―≠ΜΖ÷–ΧΪ―τΡήΉν÷’ΉΣΜ·ΈΣ________Θ§÷ς“Σ–ηΫβΨωΒΡΈ Χβ «____________ΓΘ
 –Γ―ßΫΧ≤Ρ»Ϊ≤βœΒΝ–¥πΑΗ
–Γ―ßΫΧ≤Ρ»Ϊ≤βœΒΝ–¥πΑΗ –Γ―ß ΐ―ßΩΎΥψΧβΩ®Ά―ΩΎΕχ≥ωœΒΝ–¥πΑΗ
–Γ―ß ΐ―ßΩΎΥψΧβΩ®Ά―ΩΎΕχ≥ωœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΕΰΖζΦΉΆι «–‘Ρή”≈“λΒΡΜΖ±Θ≤ζΤΖΘ§ΥϋΩ…Χφ¥ζΡ≥–©ΜαΤΤΜΒ≥τ―θ≤ψΒΡΓΑΖζάϊΑΚΓ±≤ζΤΖΘ§”ΟΉςΩ’ΒςΓΔ±υœδΚΆάδΕ≥ΩβΒ»÷–ΒΡ÷ΤάδΦΝΓΘ ‘≈–ΕœΕΰΖζΦΉΆιΒΡΚΥ¥≈Ι≤’ώ«βΤΉΙ≤”–Εύ…ΌΗωΖε(ΓΓΓΓ)
AΘ°4 BΘ°3 CΘ°2 DΘ°1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
“―÷Σ ΒΡΦϋœΏ ΫΩ…–¥Ής
ΒΡΦϋœΏ ΫΩ…–¥Ής Θ§Ρ≥”–ΜζΈοΒΡΦϋœΏ ΫΈΣ
Θ§Ρ≥”–ΜζΈοΒΡΦϋœΏ ΫΈΣ Θ§Τδ’ΐ»ΖΟϊ≥Τ «(ΓΓΓΓ)
Θ§Τδ’ΐ»ΖΟϊ≥Τ «(ΓΓΓΓ)
AΘ°5““Μυ2ΦΚœ© BΘ°2ΦΉΜυΗΐœ©
CΘ°3ΦΉΜυ5Ηΐœ© DΘ°5ΦΉΜυ2Ηΐœ©
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
«ύΤΜΙϊ÷≠”ωΒβ»ή“Κœ‘άΕ…ΪΘ§ λΤΜΙϊ÷≠ΡήΜΙ‘≠“χΑ±»ή“ΚΘ§’βΥΒΟς(ΓΓΓΓ)
AΘ°«ύΤΜΙϊ÷–÷ΜΚ§ΒμΖέ≤ΜΚ§Χ«άύ
BΘ° λΤΜΙϊ÷–÷ΜΚ§Χ«άύ≤ΜΚ§ΒμΖέ
CΘ°ΤΜΙϊΉΣ λ ±ΒμΖέΥ°ΫβΈΣΒΞΧ«
DΘ°ΤΜΙϊΉΣ λ ±ΒΞΧ«ΨέΚœ≥…ΒμΖέ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
AΓΔBΓΔCΓΔDΕΦ «÷ΜΚ§ΧΦΓΔ«βΓΔ―θ»ΐ÷÷‘ΣΥΊΒΡ”–ΜζΈοΘ§‘Ύ≥ΘΈ¬œ¬AΈΣΤχΧ§Θ§BΓΔCΈΣ“ΚΧ§Θ§D «ΑΉ…ΪΨßΧεΓΘAΓΔBΨυ”–«ΩΝ“¥ΧΦΛ–‘ΤχΈΕΘ§C”–œψΈΕΘ§D”–ΧπΈΕΓΘΥϋΟ«ΨΏ”–œύΆ§ΒΡ Β―ι ΫΘ§Ζ÷±π‘Ύ―θΤχ÷–≥δΖ÷»Φ…’ΚσΘ§Μ÷Η¥ΒΫ “Έ¬œ¬Θ§Τδ»Φ…’ΥυœϊΚΡΒΡ―θΤχΒΡΈο÷ ΒΡΝΩ”κ»Φ…’Υυ≤ζ…ζΒΡΤχΧεΒΡΈο÷ ΒΡΝΩœύΒ»ΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)…œ ω”–ΜζΈοΒΡ Β―ι Ϋ «______________ΓΘ
(2)AΒΡΫαΙΙΦρ Ϋ «____________Θ§BΒΡΫαΙΙΦρ Ϋ «____________Θ§CΒΡΫαΙΙΦρ Ϋ «______________Θ§DΒΡΫαΙΙΦρ Ϋ «____________Μρ____________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
œ¬Ν–»»Μ·―ßΖΫ≥Χ Ϋ’ΐ»ΖΒΡ «(ΓΓΓΓ)
AΘ°ΦΉΆιΒΡ±ξΉΦ»Φ…’»»ΈΣ890.3 kJΓΛmolΘ≠1Θ§‘ρΦΉΆι»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΩ…±μ ΨΈΣCH4(g)ΘΪ2O2(g)===CO2(g)ΘΪ2H2O(g)ΓΓΠΛHΘΫΘ≠890.3 kJΓΛmolΘ≠1
BΘ°500 ΓφΓΔ30 MPaœ¬Θ§ΫΪ0.5 mol N2ΚΆ1.5 mol H2÷Ο”ΎΟή±’»ίΤς÷–≥δΖ÷Ζ¥”Π…ζ≥…NH3(g)Θ§Ζ≈»»19.3 kJΘ§Τδ»»Μ·―ßΖΫ≥Χ ΫΈΣN2(g)ΘΪ3H2(g)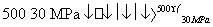 2NH3(g)ΓΓΠΛHΘΫΘ≠38.6 kJΓΛmolΘ≠1
2NH3(g)ΓΓΠΛHΘΫΘ≠38.6 kJΓΛmolΘ≠1
CΘ°“―÷Σ‘Ύ120 ΓφΓΔ101 kPaœ¬Θ§1 g H2»Φ…’…ζ≥…Υ°’τΤχΖ≈≥ω121 kJ»»ΝΩΘ§Τδ»»Μ·―ßΖΫ≥Χ ΫΈΣH2(g)ΘΪ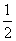 O2(g)===H2O(g)ΓΓΠΛHΘΫΘ≠242 kJΓΛmolΘ≠1
O2(g)===H2O(g)ΓΓΠΛHΘΫΘ≠242 kJΓΛmolΘ≠1
DΘ°25 ΓφΘ§101 kPa ±Θ§«ΩΥα”κ«ΩΦνΒΡœΓ»ή“ΚΖΔ…ζ÷–ΚΆΖ¥”ΠΒΡ÷–ΚΆ»»ΈΣ57.3 kJΓΛmolΘ≠1Θ§ΝρΥα»ή“Κ”κ«β―θΜ·ΦΊ»ή“ΚΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣH2SO4(aq)ΘΪ2KOH(aq)===K2SO4(aq)ΘΪ2H2O(l)ΓΓΠΛHΘΫΘ≠57.3 kJΓΛmolΘ≠1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
“‘œ¬œ÷œσ”κΚΥΆβΒγΉ”ΒΡ‘Ψ«®”–ΙΊΒΡ « (ΓΓΓΓ)ΓΘ
ΔΌΡόΚγΒΤΖΔ≥ω”–…ΪΙβΓΓΔΎάβΨΒΖ÷ΙβΓΓΔέΦΛΙβΤς≤ζ…ζΦΛΙβΓΓΔή ·”Ά’τΝσΓΓΔίΆΙΆΗΨΒΨέΙβΓΓΔό»ΦΖ≈ΒΡ―φΜπΓΔ‘Ύ“ΙΩ’÷–≥ œ÷Έε≤ γΆΖΉΒΡάώΜ®ΓΓΔΏ»’ΙβΒΤΆ®ΒγΖΔΙβΓΓΔύά以ΫαΨß
AΘ°ΔΌΔέΔόΔΏ BΘ°ΔΎΔήΔίΔύ
CΘ°ΔΌΔέΔίΔόΔΏ DΘ°ΔΌΔΎΔέΔίΔόΔΏ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
œ¬Ν–―Γœν÷–ΡήΖΔ…ζΖ¥”ΠΘ§«“ΦΉΉιΈΣ»Γ¥ζΖ¥”ΠΘ§““ΉιΈΣΦ”≥…Ζ¥”ΠΒΡ «
(ΓΓΓΓ)ΓΘ
| ΦΉ | ““ | |
| A | ±Ϋ”κδεΥ° | ““œ©”κΥ°÷Τ““¥Φ(¥ΏΜ·ΦΝ) |
| B | ”Ά÷§Υ°Ϋβ(¥ΏΜ·ΦΝΓΔΦ”»») | ±Ϋ”κ«βΤχ(¥ΏΜ·ΦΝΓΔΦ”»») |
| C | ΒμΖέΥ°Ϋβ÷ΤΤœ Χ―Χ«(¥ΏΜ·ΦΝ) | ““ΥαΚΆ““¥ΦΒΡθΞΜ· Ζ¥”Π(¥ΏΜ·ΦΝΓΔΦ”»») |
| D | ““œ©”κδεΒΡΥΡ ¬»Μ·ΧΦ»ή“Κ | ΦΉΆι”ꬻΤχ(Ιβ’’) |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
œ¬Ν–ΙΊ”ΎΈο÷ –‘÷ ΒΡ–π ω÷–Θ§’ΐ»ΖΒΡ «(ΓΓΓΓ)
AΘ°Cl2Ρή”κΫπ τΜνΕ·Υ≥–ρ±μ÷–¥σΕύ ΐΫπ τΖ¥”Π
BΘ°N2 «¥σΤχ÷–ΒΡ÷ς“Σ≥…Ζ÷÷°“ΜΘ§άΉ”ξ ±Θ§Ω…÷±Ϋ”ΉΣΜ·ΈΣNO2
CΘ°Νρ «“Μ÷÷Β≠ΜΤ…ΪΒΡΡή»ή”ΎΥ°ΒΡΨßΧεΘ§Φ»”–―θΜ·–‘”÷”–ΜΙ‘≠–‘
DΘ°Ιη «”Π”ΟΙψΖΚΒΡΑκΒΦΧε≤ΡΝœΘ§≥ΘΈ¬œ¬Μ·―ß–‘÷ ΜνΤΟ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com