
�����£���20 mL x mol��L��1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵������ȷ����
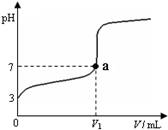
A������ CH3COOH��Һ�У�c(H+)��1��10��3 mol��L��1
B��ͼ��V1 ��20 mL
C��a���Ӧ����Һ�У�c (CH3COO��)��c (Na+��
D��������NaOH��Һ�����Ϊ20 mLʱ����Һ�У�c (CH3COOH) + c (H+)��c (OH����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ�и�����ѧ�ڵ����ε��п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
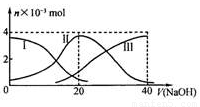
��˫ѡ�������£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ������I����H2A��II����HA����III����A2����������ͼʾ�жϣ�����˵����ȷ����

A����V(NaOH)=20mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)>c(HA��)>c(H+)>c(A2��)>c(OH��)
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�Ĵ�
C��NaHA��Һ�У�c(OH��)��c(A2��)=c(H+)��c(H2A)
D����Na2A��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ������ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й������ʵ����仯����ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�ж�����˵����ȷ����

A����y��NaOH��=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��
c(Na+) >c��HAһ��>c��H+��> c��A2- ��>c��OH����
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
C����ʹNaHA��Һ�����ԣ����������м�������
D����NaHA��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����4��˫����ϰ��ѧ�Ծ��������棩 ���ͣ�ѡ����
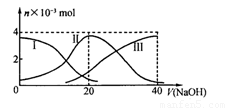
�����£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ������I����H2A��II����HA����III����A2����������ͼʾ�жϣ�����˵����ȷ����
A����V��NaOH��=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)>c(HA¯��>c(H+)> c(A2��)>c(OH¯)
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ�� ˮ�ĵ���̶ȱȴ�ˮ�Ĵ�
C��NaHA��Һ�У�c(OH��)��c(A2�C)��c(H+)��c(H2A)
D����Na2A��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ�ɶ��и�����һ������Լ�⣨���ۣ���ѧ���� ���ͣ�ѡ����
�����£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�жϣ�����˵����ȷ���� �� ��
A����y ��NaOH��=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)>cHAһ��>c��H+��>
c��A2->c��OH����
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
C����ʹNaHA��Һ�����ԣ����������м�������
D����NaHA��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com