
 Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g�� Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�����
Cu3P2��s��+3H2���ɷ���ʽ��֪�������ص���������Ԫ�����������ݹ������ؼ���PH3�����ʵ������������������ʵ�����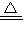 Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ���������������
Cu+H2O���ɷ���ʽ��֪������ٵ�����Ϊ����ͭʧȥ��Ԫ��������ʧȥ�����������������ˮ����2֧�Թ�����������ԭ��������������͵�1֧�Թ������ɵ������������������ټ����2֧�Թ����������ʵ�������ȥ1֧�Թ������ɵ�������Ϊԭ���������������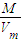 �����ܶȣ�
�����ܶȣ�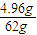 ×2mol=0.16mol��
×2mol=0.16mol��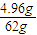 ×3mol=0.24mol��
×3mol=0.24mol��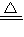 Cu+H2O ���ٵ�����
Cu+H2O ���ٵ�����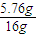 ×1mol=0.36mol��
×1mol=0.36mol��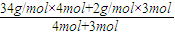 =
=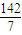 mol/L=20.3g/mol
mol/L=20.3g/mol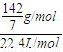 =0.91g/L
=0.91g/L


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�038
ʹһ����������������Ļ����������ͨ����֧���ȵ�Ӳ�ʲ����ܣ���һ֧��������װ��ͭм���ڶ�֧��������װ������ͭ����һ֧�����������ڷ������µķ�Ӧ��2PH![]() +3Cu
+3Cu![]() Cu
Cu![]() P
P![]() (��)+3H2
(��)+3H2![]() �����������ʵ���������4.96g���ڶ�֧�����������ʵ�����������Ӧ�������5.76g��
�����������ʵ���������4.96g���ڶ�֧�����������ʵ�����������Ӧ�������5.76g��
(1)����ԭ������������������������ȡ�
(2)�ڱ�״���£�ԭ���������ܶ��Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ƹ��ص���ҵ��������ѧ���£� ���ͣ�038
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com