
��ͼ��Ԫ�����ڱ���һ���֣����еĢ�~����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش�
 �� ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | | | | | �� | | �� | |
| �� | �� | �� | �� | �� | | | �� | �� |
| �� | �� | | | | | | �� | |
(ÿ��1�֣���10��)
��1��Ar�ṹʾ��ͼ�� ��2��F Al (3) ��4��HClO4 KOH Al(OH)3
��4��HClO4 KOH Al(OH)3
(5)Cl-�� F- ��Na+ ��Mg2+ (6) 34 H2S
���������������Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
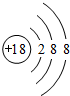
��1������Ԫ����ֻ��Ar������������Ϊ8�����ʲ����ã����ȶ�����ԭ�ӽṹʾ��ͼΪ ��
��
��2���õ���������ǿ����F���ؿ���Ԫ�صĺ����϶����O��Si��Al��Ca�������Ԫ������ΪAl��
��3��������γɵĻ�����ΪMgF2��Ϊ���ӻ���������ʽΪ ������MgF2���γɹ�����
������MgF2���γɹ����� ��
��
��4������Ԫ��������������Ӧ��ˮ������������ǿ��ΪHClO4��������ǿ��ΪKOH�������Ե���������ΪAl��OH��3���ʴ�Ϊ��HClO4��KOH��Al��OH��3��
��5���ڢۢܢ��γɵļ����Ӱ뾶�Ĵ�СΪCl-�� F- ��Na+ ��Mg2+��
��HxR�����ڱ�״���µ������5.6L��˵�����ʵ���Ϊ5.6/22.4=0.25mol������Ϊ��������Ϊ8.5g������Ħ������Ϊ8.5/0.25=34���HxR����Է�������Ϊ34��R�ڸ��⻯���е���������Ϊ94%������R��ԭ����Ϊ34*0.94=31.96=32������R�������HxR�Ļ�ѧʽΪH2S��
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
���������⿼��Ԫ�����ڱ���Ԫ�������ɣ���ϤԪ�������ڱ��е�λ���ǽ����Ĺؼ����ѶȲ���ע�⻯ѧ�����ʹ�������


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |



| ʵ�鲽�� | ʵ������ |
| 1����һС��ܵĵ��ʷ�����з�̪��Һ����ˮ�� | �Ƹ���ˮ���ϣ��۳�С���Ĵ��ζ� �Ƹ���ˮ���ϣ��۳�С���Ĵ��ζ� ������˻˻����������ʧ����Һ��ɺ�ɫ |
| 2����������ˮע��ʢ�д�ĥ���Ģݵĵ��ʵ��Թ��У��ٵμӷ�̪��һ��ʱ������������ | ���Ⱥ� þ�����������ɫ���ݣ���Һ�ʺ�ɫ þ�����������ɫ���ݣ���Һ�ʺ�ɫ |
| 3����2mL 1mol/L�������ʢ�д�ĥ���Ģݵĵ��ʵ��Թ��� | ���ҷ�Ӧ��Ѹ�ٲ���������ɫ���� |
| 4����2mL 1mol/L�������ʢ�д�ĥ���� Al Al �ĵ��ʵ��Թ��� |
��Ӧ������һ��ʱ�������ɫ���� |
| ���ۣ� Na��Mg��Al����Ԫ�صĽ��������μ��� Na��Mg��Al����Ԫ�صĽ��������μ��� ����Ԫ�ط��Ž������˵���� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
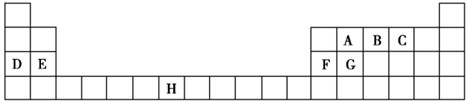
| A | |||||||||||||||||
| B | C | D | |||||||||||||||
| E | F | G | |||||||||||||||
| H | |||||||||||||||||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� ���� |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com