

| �ܶȣ�g/cm3�� | �۵� | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cu+HNO3��Ũ����Cu��NO3��2 | |||||
| B��Cu+HNO3��ϡ����Cu��NO3��2 | |||||
C��Cu
| |||||
D��Cu
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A������CuCO3���CuOҲ�ɵ�����Һ��pH����Ӱ��ʵ���� |
| B��������з�������Ҫ��ӦΪ��H2O2+Fe2++2H+�TFe3++2H2O |
| C��ϴ�Ӿ��壺���˳������©���м�����ˮ����û���壬����Ȼ���º��ظ�2��3�� |
| D������240mL 1mol/L��CuSO4��Һ�������CuSO4?5H2O������Ϊ62.5g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

| ���� | ���� | ���ͣ������ӷ���ʽ��ʾ�� |
| �μ�KSCN��Һ����Һ ______����䡱���䡱����ɫ | ______����ܡ����ܡ���ȷ����Ӧ��Ĺ��������к���+3�۵�Fe | ��______ ��______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������a��e��e��b��d��c | B���������c��d��e��e��a |
| C���������a��e��b��a��d��c | D���������b��e��a��d��c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
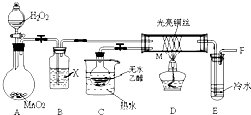
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| �����ʵ�� | ���Լ� | ��������� | �� | ||
| �� | �� | �� | |||
| �ٱ������ӣ� | A��ˮ B��NaCl���� C��NaOH��Һ D��CaO | a������ b����Һ c������ d������ | �� | ______ | ______ |
| ���Ҵ���ˮ�� | �� | ______ | ______ | ||
| �۷��������͡�ˮ�� | �� | ______ | ______ | ||
| �������������ᡢ���ᣩ | �� | ______ | ______ | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2SO4��HCl��K2S��NaOH��CO2 |
| B��NaCl��Na2SO4��Na2S��Na2OH��CO2 |
| C��NaCl��Na2SO4��NaS��NH3?H2O��HCl |
| D��Na2S��Na2SO4��NaCl��NaOH��CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com